Natural Variation Reveals Interplay between C4 Biology and Water Use Efficiency
The year 2016 marked a half-century since the discovery of C4 photosynthesis, yet we still seek to elucidate many of the mechanisms underpinning the C4 cycle. Although C4 and C3 plants share molecular units involved in photosynthesis (Miyao, 2003; Kellogg, 2013), C4 plants have unique morphological traits enabling them to efficiently convert captured CO2 into sugars at high light intensities and temperatures, conditions that are often associated with low water availability. The impetus to understand and manipulate the C4 cycle is ever greater as temperatures rise, water availability in arable lands becomes scarce, and the efficiency in crop productivity declines (Long and Ort, 2010; Long et al., 2015).
Most C4 plants are equipped with special cells, known as bundle sheath cells, that abound with chloroplasts and Rubisco and accommodate high expression of photosynthesis-related genes (Lundgren et al., 2014). Additionally, C4 plants have high numbers of plasmodesmata facilitating the movement of carbon from mesophyll cells into the bundle sheath cells. This collection of traits supercharges the photosynthetic machinery of C4 plants by containing CO2 diffusion and restricting the concomitant loss of carbon due to photorespiration, which is often a limiting factor for C3 photosynthesis. Although many genes associated with C4 photosynthesis have been identified, very few cis-elements or transfactors that control these genes are known. Thus, there are significant gaps in the knowledge required to rationally engineer C4 photosynthesis into crops. Acquiring more information on C4 photosynthesis begs for the generation of a C4 plant population with great variation in intraspecific traits related to the C4 cycle; however, generation of such a population has not been a trivial task.
In the June 2018 issue of Plant Physiology, Reeves et al. (2018) report significant natural variation within the C4 species Gynandropsis gynandra. This work should enable the identification of novel physiological characteristics of C4 plants, and this species shows great potential for in-depth investigation of C4 photosynthesis. The authors collected several accessions of G. gynandra and categorized them according to their geographic and phylogenetic location into two groups, designated as either Asian or African. The two G. gynandra groups show great variation in Kranz anatomy, with the Asian accessions having lower vein density and larger bundle sheath cell area surrounding the leaf veins. Surprisingly, G. gynandra accessions did not differ in photosynthetic performance, but rather they displayed differences in stomatal conductance that was in turn mirrored in water use efficiency, defined as the amount of dry matter produced per unit of water transpired through stomata. The observed lack of differences on CO2 assimilation rates might be due to the light conditions where the G. gynandra accessions were grown, as has been suggested previously for Arabidopsis stomatal patterning mutants (Schlüter et al., 2003).
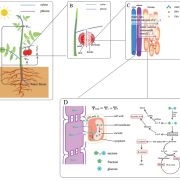
Reeves et al. (2018) demonstrated that the variation in G. gynandra species is associated with transcriptional changes in genes involved in the C4 cycle, including those encoding phosphoenolpyruvate carboxylase, the bundle sheath-specific decarboxylase NAD-dependent malic enzyme, the small subunit of Rubisco, and pyruvate,orthophosphate dikinase. Figure 1 depicts the key findings of this study, highlighting the differences among the Asian and African G. gynandra accessions at the whole-leaf, cellular, and molecular levels.
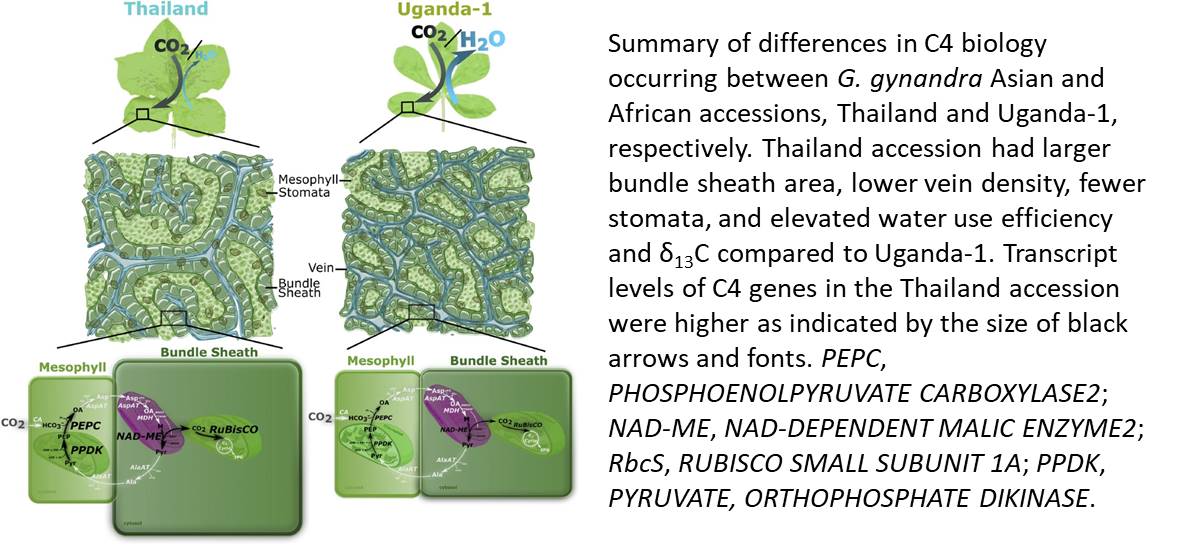
The positive correlation Reeves et al. reports between the morphological traits, especially the Kranz anatomy, and the water vapor loss reinforces the idea that targeting factors related to such traits could be useful to engineer increased water use efficiency. In fact, the authors propose that evolutionary pressure is favoring traits related to decreased water loss rather than increased photosynthesis.
Our understanding of C4 photosynthesis is far from complete, since the signaling components, including transcription factors, regulating C4 biochemical reactions, Kranz anatomy, and other C4 traits are still undetermined. However, the advancements of this study, especially the prospect of being able to map traits in a C4 species that can be easily propagated, shows potential to further dissect genomic regions regulating the C4 pathway. Finally, Reeves et al. (2018) put a new spin to the importance of C4 biology and its potential as a biotechnological strategy, by directing attention toward altering stomatal behavior to better balance water loss rather to improve the photosynthetic capacity.
REFERENCES
Kellogg EA (2013) C4 photosynthesis. Curr Biol 23: R594–R599
Long SP, Ort DR (2010) More than taking the heat: crops and global change. Curr Opin Plant Biol 13: 241–248
Long SP, Marshall-Colon A, Zhu XG (2015) Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell 161: 56–66
Lundgren MR, Osborne CP, Christin P-A (2014) Deconstructing Kranz anatomy to understand C4 evolution. J Exp Bot 65: 3357–3369
Miyao M (2003) Molecular evolution and genetic engineering of C4 photosynthetic enzymes. J Exp Bot 54: 179–189
Schlüter U, Muschak M, Berger D, Altmann T (2003) Photosynthetic performance of an Arabidopsis mutant with elevated stomatal density (sdd1-1) under different light regimes. J Exp Bot 54: 867–874