Powering Epigenetics through the 1C Pathway
By Lisa Smith and Nathan Butler
Epigenetic modifications in plants repress transposable elements to maintain genome stability and facilitate adaptation to changing environmental conditions by regulating the expression of some genes. Furthermore, conditions such as disease stress can alter the epigenetic machinery to impact gene expression and genome stability.
Addition of methyl groups to DNA and histones are two core epigenetic modifications, which are mediated by DNA methyltransferases and histone methyltransferases, respectively. Both enzymes require the metabolite S-adenosyl-Met (SAM) as a substrate (Kankel et al., 2003; Loenen, 2006). SAM is generated via the cyclic 1C pathway from Met and ATP, a reaction catalyzed by Met adenosyltransferase (MAT).
In this issue of Plant Physiology, Meng et al. (2018) isolated a mutation in one of the four Arabidopsis thaliana MAT genes (MAT4) from a genetic screen for reactivation of two epigenetically silenced transgenes. The 1C pathway has previously been implicated in epigenetics through mutation of two enzymes further removed from SAM synthesis: FPGS1 and MTHFD1 (Zhou et al., 2013; Groth et al., 2016). The mutation in MAT4 described by Meng and colleagues results in a single amino acid change. They demonstrate that knockout of MAT4 is embryonic lethal; thus, the isolated mat4 mutation is hypomorphic. Given that SAM is required for essential processes as diverse as DNA replication, cell wall formation, and chlorophyll production, it is perhaps unsurprising that loss of an enzyme that is responsible for its synthesis is lethal. The ∼35% reduction in SAM in the hypomorphic mat4 mutant is sufficient to impact plant growth, resulting in smaller seedlings.
Of the four MAT genes present in Arabidopsis, the authors show that expression of MAT4 is the most critical throughout plant development, with all MAT cDNAs capable of complementing the mat4 mutant phenotype when expressed from the MAT4 promoter. Thus, the Arabidopsis MAT enzymes have similar biological functions but different expression patterns. There was no reduction in SAM levels in mat3 mutant seedlings and only a 6% decrease in mat1 and mat2 seedlings, indicating a dominant role for mat4 during vegetative plant growth. Nonetheless, the mat3 mutant produces fewer seeds, supporting a role for MAT3 in gamete production and fertility. The MAT3 enzyme demonstrated the highest in vitro activity, possibly due to the importance of transcriptional repression/epigenetic regulation in early gamete development. The biological roles of MAT1 and MAT2 remain unknown, with mat1 mat2 double mutants exhibiting no aberrant phenotype. However, mat1 mat4 and mat2 mat4 double mutants (using hypomorphic mat4) are much smaller than normal and have embryonic defects, respectively, so MAT4 may work in combination with MAT1 and MAT2 to facilitate normal growth and early development. This hypothesis that multiple MAT genes function redundantly or in parallel is supported by the direct interaction of MAT4 with MAT1, 2, and 3 enzymes both in vitro and in vivo. MAT1 and MAT2 may be environmentally regulated, as the receptor kinase FER has been shown to decrease the expression of MAT genes in response to stress (Mao et al., 2015).
Mutation of MAT4 has a global impact on DNA and histone methylation; however, effects are variable between epigenetic modifications and genomic loci. With respect to DNA methylation, CG methylation was largely maintained, while CHG and CHH methylation were halved in the mat4 mutant. Furthermore, the histone modifications H3K9me2 and H3K27me1, both associated with suppression of transcription and heterochromatin formation, were reduced, whereas H3K9me1, which also suppresses transcription, and H3K4me3, which is associated with active transcription, remained unaltered in mat4. Addressing why some epigenetic modifications are more sensitive to a reduction in SAM than others will require further research. However, it can be speculated that the DNA and histone methyltransferases may have different affinities for SAM, with those displaying higher affinity being less affected by a reduction in SAM. Thus, a further reduction in SAM beyond that observed in mat4 may have a greater effect on epigenetic modifications, such as CG methylation and H3K4me3.
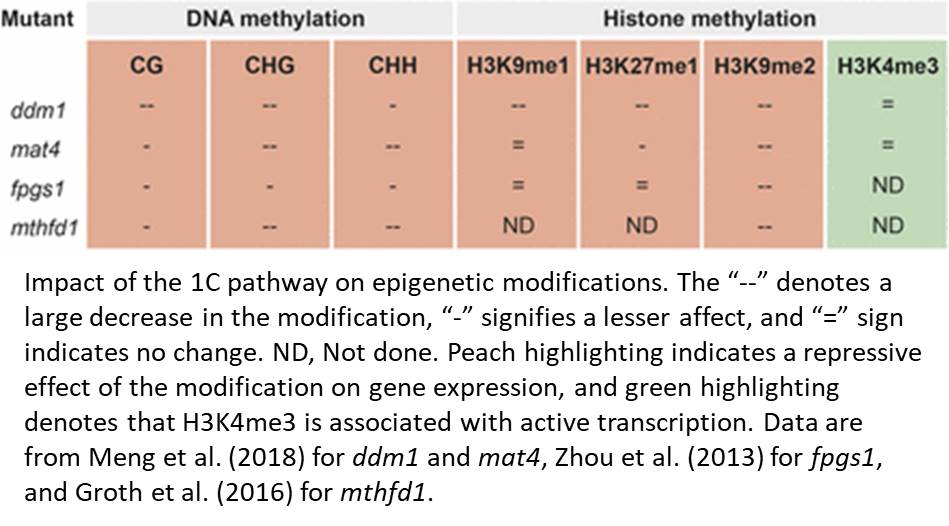
Meng and colleagues compared the phenotype of their mat4 mutant to that of other mutants with a loss of proteins involved in DNA methylation and histone modifications, including the chromatin remodeler DDM1 and the two other 1C metabolic proteins that have previously been shown to regulate epigenetic activity in Arabidopsis (FPGS1 and MTHFD1; Zhou et al., 2013; Groth et al., 2016). A comparison of how ddm1, mat4, fpgs1, and mthfd1 mutations affect DNA and histone methylation (Fig. 1) reveals that while DDM1 mostly controls symmetric (CG and CHG) DNA methylation and repressive histone methylation, the 1C pathway predominantly affects CHG and CHH methylation along with H3K9me2. H3K4me3 levels are not altered by any of the 1C pathway mutants. The hypomorphic nature of the mat4 mutation may also explain why it has less of an impact on DNA and histone modifications than the ddm1 mutation isolated in parallel from the genetic screen.
Why some genomic regions are more strongly affected than others is a harder question to address. As the 35S promoter that drives kanamycin resistance has a stronger reduction in H3K9me2 methylation but only a very modest reduction in DNA methylation in mat4, transcriptional release of the 35S promoter likely arises either through reduction in a single histone modification or through an indirect pathway. While a direct effect on DNA and histone methylation levels is to be expected when levels of SAM are reduced, indirect effects are also possible. Indeed, a feedback loop between DNA methylation and histone modifications has been described (Matzke et al., 2015). Therefore, if histone methyltransferases were more sensitive to a reduction in SAM, a loss of histone methylation would indirectly result in lowering DNA methylation levels and vice versa. Indirect effects may also be mediated through metabolite changes, with the mat4 mutant having elevated levels of S-adenosyl-homo-Cys SAH, an intermediate of the 1C pathway that competes with SAM for binding to MAT and acts as an inhibitor. Why SAH levels are higher in mat4 is unclear. Additionally, Meng and colleagues note that expression of a suppressor of DNA methylation (SDC) is increased by the mat4 mutation, highlighting another route by which DNA methylation levels could be indirectly impacted by a reduction in SAM.
While the study by Meng and colleagues provides exciting new insight into the metabolic regulation of epigenetic plant activity, further research is required to ascertain to what extent the 1C metabolic cycle is connected directly and/or indirectly to the DNA and histone methylation pathways. Given the lack of change in CG and H3K4me3 methylation, not all epigenetic modifications are equally affected by a reduction in SAM. This presents two plausible explanations. Indirect effects may impact specific methyltransferases, or, more likely, the direct effect differs due to the specific kinetics and substrate (SAM and SAH) affinities of each methyltransferase. A closer look at the biochemical properties of the methyltransferases and an understanding of the biological conditions under which SAM levels become limiting could shed light on the integration of metabolic pathways with epigenetics in plants.
REFERENCES
Groth M, Moissiard G, Wirtz M, Wang H, Garcia-Salinas C, Ramos-Parra PA, Bischof S, Feng S, Cokus SJ, John A, et al. (2016) MTHFD1 controls DNA methylation in Arabidopsis. Nat Commun 7: 11640
Kankel MW, Ramsey DE, Stokes TL, Flowers SK, Haag JR, Jeddeloh JA, Riddle NC, Verbsky ML, Richards EJ (2003) Arabidopsis MET1 cytosine methyltransferase mutants. Genetics 163: 1109–1122
Loenen WAM (2006) S-Adenosylmethionine: Jack of All Trades and Master of Everything? Portland Press Limited, London
Mao D, Yu F, Li J, Van de Poel B, Tan D, Li J, Liu Y, Li X, Dong M, Chen L, Li D, Luan S (2015) FERONIA receptor kinase interacts with S-adenosylmethionine synthetase and suppresses S-adenosylmethionine production and ethylene biosynthesis in Arabidopsis. Plant Cell Environ 38: 2566–2574
Matzke MA, Kanno T, Matzke AJ (2015) RNA-directed DNA methylation: the evolution of a complex epigenetic pathway in flowering plants. Annu Rev Plant Biol 66: 243–267
Zhou H-R, Zhang F-F, Ma Z-Y, Huang H-W, Jiang L, Cai T, Zhu J-K, Zhang C, He X-J (2013) Folate polyglutamylation is involved in chromatin silencing by maintaining global DNA methylation and histone H3K9 dimethylation in Arabidopsis. Plant Cell 25: 2545–2559