An epigenetic origin behind the transgenerational fitness decline in chromatin assembly impaired plants (New Phytol) ($)
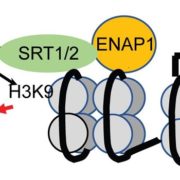
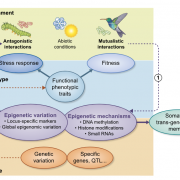
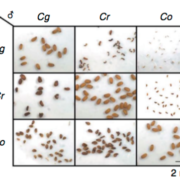
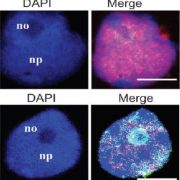
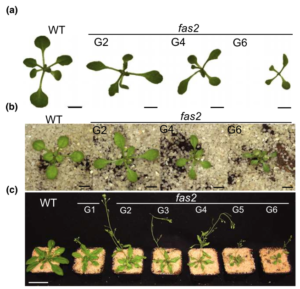 Packaging of nuclear DNA in chromatin is critical for the maintenance of genome integrity as well as fundamental cellular processes such as DNA replication and transcription. Chromatin assembly is mediated by histone chaperones, such as Chromatin Assembly Factor 1 (CAF-1). CAF-1 ensures faithful replication-coupled chromatin assembly. Arabidopsis plants lacking a functional CAF-1 show molecular and developmental defects of increasing intensity after repeated propagation by selfing. Nevertheless, the molecular basis of this rise in phenotype severity remains unclear. In this study, Mozgova et al. monitored the transgenerational phenotypic aggravation of caf-1 mutants and associated changes in the transcriptome and methylome. They first confirmed the progressive aggravation of phenotypic defects through caf-1 generations, and pinpointed defects in ovule development as a major cause of sterility in late caf-1 generations. By comparing the transcriptome of early vs. late generation of caf-1 mutants, they further showed changes in transcription in defense-related genes, mirroring the transgenerational aggravation of the phenotype. Methylome analysis revealed changes in DNA methylation at transposable elements through generations, while genes remained globally unaffected. Remarkably, reciprocal crosses between early (G1) and late (G4) caf-1 generations argued for a major maternal contribution to the defective phenotype. Together, this study provides new insights in the molecular basis of phenotypic aggravation in plants lacking CAF-1, and stresses out the importance of replication-coupled chromatin assembly in the faithful transmission of the epigenome and the transcriptional program. (Summary by Matthias Benoit) New Phytol. 10.1111/nph.15082
Packaging of nuclear DNA in chromatin is critical for the maintenance of genome integrity as well as fundamental cellular processes such as DNA replication and transcription. Chromatin assembly is mediated by histone chaperones, such as Chromatin Assembly Factor 1 (CAF-1). CAF-1 ensures faithful replication-coupled chromatin assembly. Arabidopsis plants lacking a functional CAF-1 show molecular and developmental defects of increasing intensity after repeated propagation by selfing. Nevertheless, the molecular basis of this rise in phenotype severity remains unclear. In this study, Mozgova et al. monitored the transgenerational phenotypic aggravation of caf-1 mutants and associated changes in the transcriptome and methylome. They first confirmed the progressive aggravation of phenotypic defects through caf-1 generations, and pinpointed defects in ovule development as a major cause of sterility in late caf-1 generations. By comparing the transcriptome of early vs. late generation of caf-1 mutants, they further showed changes in transcription in defense-related genes, mirroring the transgenerational aggravation of the phenotype. Methylome analysis revealed changes in DNA methylation at transposable elements through generations, while genes remained globally unaffected. Remarkably, reciprocal crosses between early (G1) and late (G4) caf-1 generations argued for a major maternal contribution to the defective phenotype. Together, this study provides new insights in the molecular basis of phenotypic aggravation in plants lacking CAF-1, and stresses out the importance of replication-coupled chromatin assembly in the faithful transmission of the epigenome and the transcriptional program. (Summary by Matthias Benoit) New Phytol. 10.1111/nph.15082