MYB30 Regulates Photomorphogenesis via Interactions with Active Phytochromes and PIFs
MYB proteins are a group of transcription factors that are highly conserved in all vertebrates and were first implicated in avian myeloblastosis (leukemia). Subsequently, they were shown to be cellular proto-oncogenes that regulate production of blood cells in animals. MYB proteins contain a DNA-binding MYB domain, which consists of one or more imperfect repeats of a sequence of approximately 50 amino acid residues.
Members of the R2R3 subfamily, such as Arabidopsis MYB30, are characterized by the presence of two MYB domains in the N-terminal region of the protein plus a variable C-terminal regulatory region. MYB30 participates in a variety of plant developmental, defense, and stress responses such as the hypersensitive response to plant pathogens, tolerance to salt and reactive oxygen species, and responses to heat and oxidative stress (Mabuchi et al., 2018). It also integrates these responses with other biochemical pathways in plants including phytohormone signaling, fatty acid metabolism, and calcium signaling.
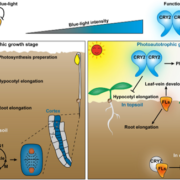
Yan et al. (2020) now show that MYB30 regulates photomorphogenesis, the light-induced developmental pathway responsible for inhibition of hypocotyl growth of the germinating seedling as it reaches the soil surface and perceives light. MYB30 not only promoted hypocotyl growth, thus acting as a negative regulator of photomorphogenesis, but also bound to both phytochrome A (phyA), phyB and two phytochrome-interacting factors (PIF4 and PIF5).
 In yeast two-hybrid analyses, all six members of the MYB30 clade of the Arabidopsis R2R3 subfamily interacted with the phyA C-terminus, but only MYB30 strongly interacted with the phyB C-terminus. After adding the chromophore phycocyanobilin to yeast cells and irradiating them with either far-red (FR) light or FR followed by red (R) light, MYB30 preferentially interacted with the active Pfr form of both phyA and phyB.
In yeast two-hybrid analyses, all six members of the MYB30 clade of the Arabidopsis R2R3 subfamily interacted with the phyA C-terminus, but only MYB30 strongly interacted with the phyB C-terminus. After adding the chromophore phycocyanobilin to yeast cells and irradiating them with either far-red (FR) light or FR followed by red (R) light, MYB30 preferentially interacted with the active Pfr form of both phyA and phyB.
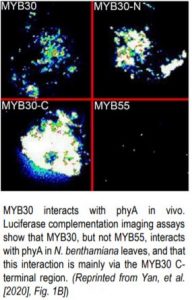
The in vivo interaction of phyB and MYB30 was supported by coimmuno-precipitation experiments using phyB-mCherry, MYB30-GFP, and an anti-GFP antibody. Luciferase complementation imaging assays in Nicotiana benthamiana leaves confirmed that MYB30 and phyA interacted in leaves, primarily via the MYB30 C-terminal region (see figure).
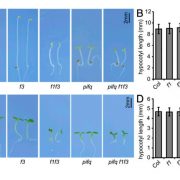
To better understand the role of MYB30 in regulation of photomorphogenesis, they grew seedlings of wild-type Col-0 and two myb30 mutants either in darkness or in continuous white (W), R, FR or blue (B) light. Both myb30 mutants had significantly inhibited hypocotyl growth compared to Col-0 in all conditions but FR. Introduction of MYB30-GFP driven by the native MYB30 promoter rescued this defect in all of the conditions except FR, verifying that the short-hypocotyl phenotype of the myb30 mutants was due to loss of MYB30.
Curiously, although MYB30 transcript levels were higher in darkness than in all four light conditions in both RT-qPCR and GUS assays, MYB30 itself accumulated to much higher levels in the light, suggesting increased protein stability. Also, MYB30 accumulated rapidly within 1 h of W- or R-light irradiation and was degraded when W or R light-grown seedlings were transferred to darkness for 24 h. Because MYB30 interacted directly with both phyA and phyB, they used a phyA phyB double mutant and showed phytochrome-dependent regulation of light-induced MYB30 accumulation.
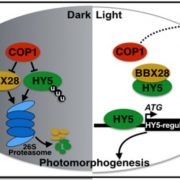
In a search for target genes of MYB30, they found that the expression of both PIF4 and PIF5, which are negative regulators of photo-morphogenesis, was induced by MYB30 following prolonged R light irradiation. Both PIF4 and PIF5 promoters contain putative MYB-binding sites and, using yeast one-hybrid and electrophoretic mobility shift assays in combination with chromatin immuno-precipitation assays with extracts from MYB30-overexpressing seedlings, the authors showed that MYB30 binds to the promoters of both PIFs in vitro and in vivo. They also showed that MYB30 inhibits the interaction of PIFs with the Pfr form of phyB.
Although it has been documented that phytochromes induce rapid phosphorylation and degradation of PIFs upon R light exposure (Pham et al., 2018), we now know that MYB30 promotes PIF4 and PIF5 reaccumulation under prolonged R irradiation via both transcriptional and post-translational mechanisms. The authors conclude by presenting a compelling model proposing how MYB30 regulates photomorphogenesis by direct interactions with both phytochromes and PIFs following light irradiation.
Gregory Bertoni
Science Editor
ORCID ID: 0000-0001-7977-3724
REFERENCES
Mabuchi, K., Maki, H., Itaya, T., Suzuki, T., Nomoto, M., Sakaoka, S., Morikami, A., Higashiyama, T., Tada, Y., Busch, W., and Tsukagoshi, H. (2018). MYB30 links ROS signaling, root cell elongation, and plant immune responses. Proc. Natl. Acad. Sci. USA 115: E4710-E4719.
Pham, V.N., Kathare, P.K., and Huq, E. (2018). Phytochromes and phytochrome interacting factors. Plant Physiol. 176: 1025-1038.
Yan, Y., et al. (2020). MYB30 is a key negative regulator of Arabidopsis photomorphogenic development that promotes PIF4 and PIF5 protein accumulation in the light. Plant Cell. DOI: https://doi.org/10.1105/tpc.09.00645.