Metacaspases meet stress granules
By Nerea Ruiz-Solaní1,2, Laia Armengot1,2 and Núria S. Coll1,3
1 Centre for Research in Agricultural Genomics (CRAG), CSIC-IRTA-UAB-UB, Bellaterra 08193, Spain
2 Department of Genetics, Microbiology and Statistics, Universitat de Barcelona, Barcelona 08028, Spain
3 Consejo Superior de Investigaciones Científicas (CSIC), Barcelona 08001, Spain
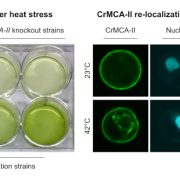
Background: Heat stress leads to protein misfolding and subsequent aggregation of misfolded proteins into cytotoxic aggregates, which may become harmful to the plant and accelerate aging, a phenomenon termed proteotoxicity. Plants have several mechanisms in place to cope with such protein aggregates, including the formation of stress granules (SGs). SGs are cytoplasmic membrane-less condensates that form under stress conditions and sequester mRNA and proteins. An essential property of SGs is their dynamism: to be functional, they must be inducible and reversible. Enzymes that break down proteins, such as the cysteine protease METACASPASE1 (MC1) may also contribute to clearing protein aggregates, but its interaction with SGs remains unclear.
Question: Does the Arabidopsis (Arabidopsis thaliana) metacaspase MC1 contribute to the clearance of protein aggregates?
Findings: We found that Arabidopsis MC1 is dynamically recruited into SGs upon proteotoxic stress such as heat stress. AtMC1 localization in SG is mediated by regions of the protein that are intrinsically disordered. Furthermore, MC1 participates in clearinh plant protein aggregates and it can also disaggregate pathological proteins in evolutionarily distant organisms ranging from yeast to animals. In plants, overexpression of AtMC1 leads to a delay in senescence.
Next steps: Despite their important role in stress responses, little is known about plant SGs. It will be interesting to determine the composition of MC1-containing SGs in the context of different stressful conditions leading to proteotoxicity. In addition, the function of MC1 may inspire further research on protein disaggregases for therapeutic intervention in age-related protein-misfolding diseases in humans and for efforts to delay aging in plants.
Reference:
Nerea Ruiz-Solaní, Jose Salguero-Linares, Laia Armengot, Jaime Santos, Irantzu Pallarès, Katarina P. van Midden, Ujjal J. Phukkan, Seda Koyuncu, Júlia Borràs-Bisa, Liang Li, Crina Popa, Frederik Eisele, Anna Maria Eisele-Bürger, Sandra Malgrem Hill, Emilio Gutiérrez-Beltrán, Thomas Nyström, Marc Valls, Ernesto Llamas, David Vilchez, Marina Klemenčič, Salvador Ventura, Nuria S. Coll (2023) Arabidopsis metacaspase MC1 localizes in stress granules, clears protein aggregates and delays senescence. https://doi.org/10.1093/plcell/koad172