Thermoprotection by a metacaspase
Zhou et al. examine the fundamental functions of metacaspases in the unicellular green alga Chlamydomonas reinhardtii.
https://doi.org/10.1093/plcell/koad289
By Yong Zou, Adrian N. Dauphinee, Simon Stael and Peter V. Bozhkov
Department of Molecular Sciences, Uppsala BioCenter, Swedish University of Agricultural Sciences and Linnean Center for Plant Biology, Uppsala, Sweden
Background: Metacaspases are a large class of proteases spanning kingdoms of life including bacteria but absent from animals; they are believed to represent an ancestral group from which animal-specific caspases evolved. In contrast to caspases, a textbook example of well-studied proteases, metacaspases remain poorly understood. Recent research just began to link metacaspases to the biology of land plants, which possess multiple metacaspase genes (e.g., nine in Arabidopsis thaliana) split into two major types (I and II), which substantially complicates their characterization. By contrast, green algae represent an ideal model for metacaspase research, as their genomes carry only one or two metacaspase genes.
Question: We wished to establish a toolbox of chemical probes and mutant strains for metacaspase research in a major unicellular green algal model, Chlamydomonas (Chlamydomonas reinhardtii). With such a toolbox in hand, we intended to investigate the fundamental functions of metacaspases in a unicellular plant lineage.
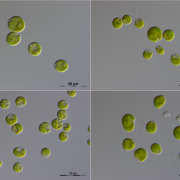
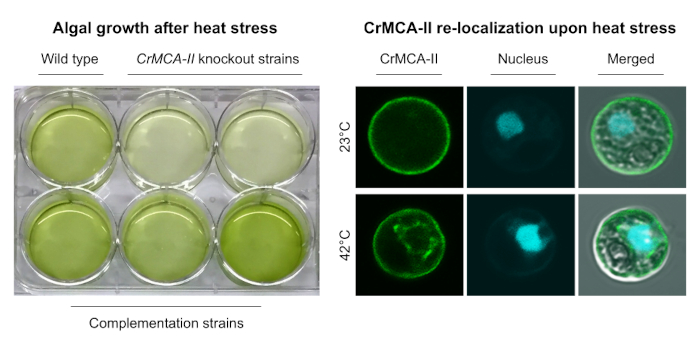 Findings: Knockout of the single type-II metacaspase gene of Chlamydomonas (named CrMCA-II) decreased algal fitness under heat stress (42°C), resulting in lower growth and increased cell death. Unlike other studied plant metacaspases, CrMCA-II was abundantly associated with the plasma membrane but partially translocated to the cytoplasm during heat treatment. Another peculiar feature of CrMCA-II was its oligomerization to form both catalytically active dimers and larger, megadalton-scale complexes comprising inactive pro-enzyme (zymogen). We discovered that the thermoprotective function of CrMCA-II is independent of its proteolytic activity but instead might be associated with an unknown mechanism by which CrMCA-II mediates fluidization of the plasma membrane.
Findings: Knockout of the single type-II metacaspase gene of Chlamydomonas (named CrMCA-II) decreased algal fitness under heat stress (42°C), resulting in lower growth and increased cell death. Unlike other studied plant metacaspases, CrMCA-II was abundantly associated with the plasma membrane but partially translocated to the cytoplasm during heat treatment. Another peculiar feature of CrMCA-II was its oligomerization to form both catalytically active dimers and larger, megadalton-scale complexes comprising inactive pro-enzyme (zymogen). We discovered that the thermoprotective function of CrMCA-II is independent of its proteolytic activity but instead might be associated with an unknown mechanism by which CrMCA-II mediates fluidization of the plasma membrane.
Next steps: Understanding exactly how CrMCA-II works at the subcellular and molecular levels to confer thermoprotection to algal cells awaits further investigations. In this context, isolation and characterization of the megadalton-size CrMCA-II complex, including its structural analysis, represents a particular challenge.
Reference:
Yong Zou, Igor Sabljić, Natalia Horbach, Adrian N. Dauphinee, Anna Åsman, Lucia Sancho Temino, Elena A. Minina, Marcin Drag, Simon Stael, Marcin Poreba, Jerry Ståhlberg and Peter V. Bozhkov (2024). Thermoprotection by a cell membrane-localized metacaspase in a green alga. https://doi.org/10.1093/plcell/koad289