Lipid Anchor: Postal Code for Proteins on the Road to Membranes
Majeran et al. investigate how plant cells target proteins to membrane compartments https://doi.org/10.1105/tpc.17.00523
By Wojciech Majeran, Thierry Meinnel & Carmela Giglione
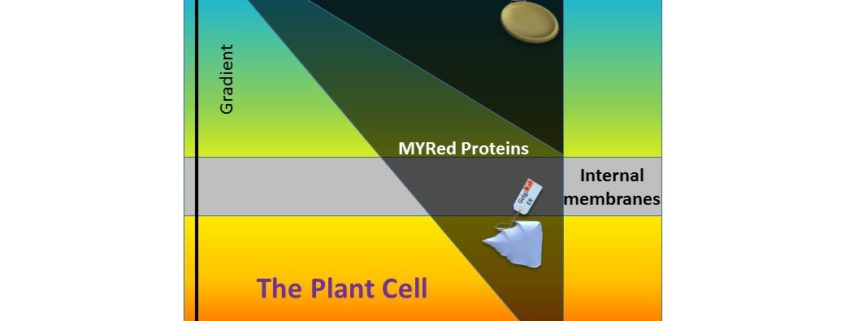
Background: Living cells are encased in an oily barrier, the plasma membrane, made up of a double layer of lipids and embedded proteins, and similar membranes surround organelles within the cells. Proteins continually move across these membranes to reach the location they need to be to function properly. Some proteins reside permanently in membranes – including those whose role is to help others get through, while others are transients – passing through en route to another location. A device that cells use to target proteins to membrane compartments is to add a small tag to the end of a protein made of a fatty acid called myristate. This tag acts as both a postal code and a lipid anchor, often in association with another lipid (palmitate) – helping to deliver a protein to the membrane and securing it there for as long as needed. Although these lipid tags are crucial for cell survival and hundreds of proteins undergo myristoylation, only a few examples have been documented experimentally, because membranes are notoriously difficult to work with.
Question: How can we detect these anchor lipids and the proteins underdoing lipid modification, and how are they distributed in different subcellular compartments?
Findings: We used mass spectrometry for massive protein identification and to assess how thousands of proteins are distributed within different cellular compartments. We first purified various compartments and set the conditions specifically to detect Myristoylated (MYR) and Palmytoylated (PAL) proteins. We found an asymmetric gradient leading most MYR proteins to the membranes and those with PAL or a positively charged sequence to the negatively-charged part of the plasma membrane.
Next Steps: We anticipate that this work will be of high interest to scientists in diverse communities since MYR is one of the most crucial protein modifications in animals and plants. Since we report localization and lipidation information for a number of important protein families, the results will influence research in many areas of cell biology, such as cell proliferation, cytoskeletal assembly, programmed cell death, root and organ shape, seedling development, protein degradation and intracellular vesicle trafficking.
Majeran, W., Le Caer, J.-P., Ponnala, L., Meinnel, T., and Giglione, C. (2018). Targeted profiling of A. thaliana sub-proteomes illuminates new co- and post-translationally N-terminal Myristoylated proteins. Plant Cell. https://doi.org/10.1105/tpc.17.00523.