Ubiquitin-dependent chloroplast-associated protein degradation in plants (Science) ($)
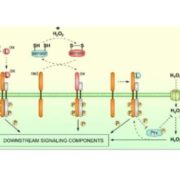
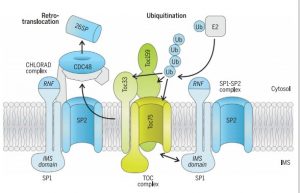 The fidelity of the chloroplast proteome is a major factor in the functional efficiency of photosynthesis. A RING-type ubiquitin E3 ligase, SP1, is an outer envelope localized protein in the chloroplast which ubiquitinates the protein import translocases (TOC proteins) and thus target them to 26S mediated degradation. In recent work, Ling et al. identified a mutation that suppresses the chlorosis phenotype of the Toc33 mutant (ppil), which they named suppressor of ppi1 locus 2 (sp2). Using genetic and protein-interaction methods, the authors confirmed that AAA+ chaperone CDC48 and SP2 protein participate in the same proteolytic pathway with SP1. Thus, they conceptualize the multicomponent system for chloroplast envelope protein removal by chloroplast-associated protein degradation (CHLORAD). (Summary by Kaushal Kumar Bhati) Science 10.1126/science.aav4467
The fidelity of the chloroplast proteome is a major factor in the functional efficiency of photosynthesis. A RING-type ubiquitin E3 ligase, SP1, is an outer envelope localized protein in the chloroplast which ubiquitinates the protein import translocases (TOC proteins) and thus target them to 26S mediated degradation. In recent work, Ling et al. identified a mutation that suppresses the chlorosis phenotype of the Toc33 mutant (ppil), which they named suppressor of ppi1 locus 2 (sp2). Using genetic and protein-interaction methods, the authors confirmed that AAA+ chaperone CDC48 and SP2 protein participate in the same proteolytic pathway with SP1. Thus, they conceptualize the multicomponent system for chloroplast envelope protein removal by chloroplast-associated protein degradation (CHLORAD). (Summary by Kaushal Kumar Bhati) Science 10.1126/science.aav4467