It’s Not Easy Not Being Green: Breakthroughs in Chlorophyll Breakdown
IN BRIEF by Jennifer Mach [email protected]
Plants can dispose of organs such as leaves and recycle the nutrients in these organs into new leaves, seeds, or storage organs. However, when separated from its photosystem proteins, chlorophyll can be phototoxic, absorbing light and producing high-energy electrons. The complex chlorophyll degradation pathway solves this problem by breaking down chlorophyll into colorless catabolites that are stored in the vacuole (reviewed in Christ and Hörtensteiner, 2014).
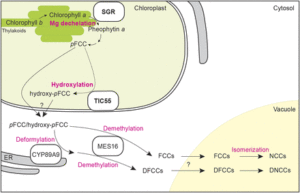
One of the first steps in chlorophyll breakdown is the removal of the Mg from chlorophyll a to form pheophytin a. Pheophytin a also functions as part of the D1/D2 complex in photosystem II, helping to drive electron transfer during photosynthesis. However, the identity (and even existence) of a putative Mg-dechelatase that removes Mg from chlorophyll a has remained unclear. In a recent Breakthrough Report, Shimoda et al. (2016) reason that mutants in Mg-dechelatase would have a stay-green phenotype; therefore, the authors examine proteins related to pea (Pisum sativum) STAY-GREEN (SGR), which causes the green-cotyledon phenotype described by Mendel. The Arabidopsis thaliana genome has three SGRs: SGR1, SGR2, and STAY-GREEN LIKE (SGRL). Recombinant SGR1 and SGR2 expressed in wheat germ extract showed high dechelating activity on chlorophyll a; by contrast, SGRL showed higher activity on chlorophyllide a. Also, expression of SGR1 caused an increase in pheophytin a in transgenic Arabidopsis plants and in cyanobacteria. Arabidopsis mutants for pheophytinase, the next enzyme in the chlorophyll breakdown pathway, also accumulated more pheophytin a when expressing SGR1. Expression of SGR1 in Arabidopsis also caused decreases in chlorophyll contents and in photosystem proteins, but not other chloroplast proteins, indicating that SGR can act on the chlorophyll in protein complexes and thus regulate the amounts of these complexes.
Chlorophyll breakdown initiates in the chloroplast, where enzymes transform pheophorbide a into primary fluorescent chlorophyll catabolite (pFCC). In the cytoplasm, pFCC undergoes species-specific modifications; all species studied also hydroxylate pFCC at the C32 position to form hydroxy-pFCC. Using chromoplasts from bell pepper (Capsicum annuum), Hauenstein et al. (2016) find that the activity that hydroxylates pFCC localizes in the plastid membrane. This activity also requires O2 and ferredoxin, similar to the Rieske-type oxygenase PHEOPHORBIDE a OXYGENASE (PAO). The Arabidopsis genome encodes five Rieske-type oxygenases; of these, three localize to chloroplast membranes: PAO, TRANSLOCON AT THE INNER CHLOROPLAST ENVELOPE55 (TIC55), and PROTOCHLOROPHYLLIDE-DEPENDENT TRANSLOCON AT THE INNER CHLOROPLAST ENVELOPE52 (PTC52). The tic55 and ptc52 mutants showed no visible phenotypes, but the tic55 mutants showed decreased levels of hydroxy-pFCC. By contrast, the ptc52 mutants showed wild-type levels of hydroxy-pFCC. Previous work showed that TIC32 and TIC62 function with TIC55, possibly delivering electrons for the TIC55 redox cycle; however, the authors observed no changes in hydroxylated phyllobilins in tic32 and tic62 mutants.
The finding that TIC55 functions in chlorophyll breakdown proved surprising because previous work implicated TIC55 in protein transport into the chloroplast, acting with TIC32 and TIC62 to sense the redox state of the chloroplast and regulate protein import. However, this finding casts doubt on its potential function in protein transport. Therefore, the roles of these intriguing chloroplast proteins, TIC55 and the SGRs, remain interesting subjects for future work, specifically whether and how SGRs regulate the protein levels of photosystem and light-harvesting complex proteins and whether TIC55 has additional functions in other aspects of chloroplast biology. Given that the tic55 mutants show no obvious visible phenotype, the importance of hydroxylation of phyllobilins also remains an outstanding question.
REFERENCES
Christ, B., and Hörtensteiner, S. (2014). Mechanism and significance of chlorophyll breakdown. J. Plant Growth Regul. 33: 4–20.
Hauenstein, M., Christ, B., Das, A., Aubry, S., and Hörtensteiner, S. (2016). A role for TIC55 as a hydroxylase of phyllobilins, the products of chlorophyll breakdown during plant senescence. Plant Cell 28: 2510–2527.
Shimoda, Y., Ito, H., and Tanaka, A. (2016). Arabidopsis STAY-GREEN, Mendel’s green cotyledon gene, encodes magnesium-dechelatase. Plant Cell 28: 2147–2160.







Leave a Reply
Want to join the discussion?Feel free to contribute!