Iron Accumulation and Fraxetin, a Coumarin
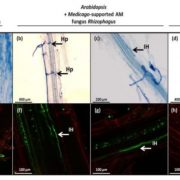
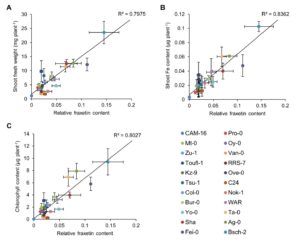 Soil pH has a strong influence on the availability of mineral nutrients and the distribution of species in natural plant communities. Iron (Fe) solubility decreases dramatically with increasing pH. In alkaline soils, calcifuge (“chalk-fleeing”) species are unable to compete due to their inability to acquire sufficient Fe. Calcicole behavior, i.e. the ability to thrive on alkaline soils, has been attributed to the efficiency of Fe acquisition of a cultivar or species, a trait that strongly contributes to the ability to compete on such soils. In Arabidopsis, the scopoletin pathway is reprogrammed upon Fe deficiency to produce and secrete coumarins with Fe-mobilizing properties. In this issue, Tsai et al. (10.1104/pp.18.00178) examine the biosynthesis and ecological role of Fe-mobilizing coumarins secreted by Fe-deficient Arabidopsis plants. More specifically, they show that scopoletin 8-hydroxylase (S8H), participates in Fe acquisition by mediating the biosynthesis of fraxetin (7,8-dihydroxy-6- methoxycoumarin), a coumarin derived from the scopoletin pathway. S8H is highly induced in roots of Fe-deficient plants. Mutants defective in the expression of S8H showed increased sensitivity to growth on pH 7 media and reduced secretion of fraxetin. Transgenic lines overexpressing S8H exhibited an opposite phenotype. Homozygous s8h mutants grown on media with immobilized Fe accumulated significantly more scopolin, the storage form of scopoletin. Supplementation of a medium containing immobile Fe with fraxetin partially rescued the s8h mutants. In natural Arabidopsis accessions differing in their performance on media containing immobilized Fe, the amount of secreted fraxetin was highly correlated with growth and Fe and chlorophyll content, indicating that fraxetin secretion is a key factor in the calcicole-calcifuge behavior of plants.
Soil pH has a strong influence on the availability of mineral nutrients and the distribution of species in natural plant communities. Iron (Fe) solubility decreases dramatically with increasing pH. In alkaline soils, calcifuge (“chalk-fleeing”) species are unable to compete due to their inability to acquire sufficient Fe. Calcicole behavior, i.e. the ability to thrive on alkaline soils, has been attributed to the efficiency of Fe acquisition of a cultivar or species, a trait that strongly contributes to the ability to compete on such soils. In Arabidopsis, the scopoletin pathway is reprogrammed upon Fe deficiency to produce and secrete coumarins with Fe-mobilizing properties. In this issue, Tsai et al. (10.1104/pp.18.00178) examine the biosynthesis and ecological role of Fe-mobilizing coumarins secreted by Fe-deficient Arabidopsis plants. More specifically, they show that scopoletin 8-hydroxylase (S8H), participates in Fe acquisition by mediating the biosynthesis of fraxetin (7,8-dihydroxy-6- methoxycoumarin), a coumarin derived from the scopoletin pathway. S8H is highly induced in roots of Fe-deficient plants. Mutants defective in the expression of S8H showed increased sensitivity to growth on pH 7 media and reduced secretion of fraxetin. Transgenic lines overexpressing S8H exhibited an opposite phenotype. Homozygous s8h mutants grown on media with immobilized Fe accumulated significantly more scopolin, the storage form of scopoletin. Supplementation of a medium containing immobile Fe with fraxetin partially rescued the s8h mutants. In natural Arabidopsis accessions differing in their performance on media containing immobilized Fe, the amount of secreted fraxetin was highly correlated with growth and Fe and chlorophyll content, indicating that fraxetin secretion is a key factor in the calcicole-calcifuge behavior of plants.