How plants and synthetic biology could help us fight diabetes
Dr. Raimund Nagel
Iowa State University
Plants produce a plethora of natural products that function as defensive compounds are frequently used by humans for medicinal purposes. A majority of these natural products, however, are either found in low concentrations, in slow growing plants, or are highly organ specific. Paclitaxel (Taxol), which is used to treat a variety of cancers, is a prominent example of such a product. In some cases, chemical synthesis is an alternative source, but natural products often contain multiple stereocenters, which can be a major roadblock for chemists. Therefore, a preferred way to produce large quantities of desired natural products is through bioengineering (Dziggel et al., 2017; Teijaro et al., 2019). Compounds with improved bioactivity could then be created either through chemical synthesis or synthetic biology.
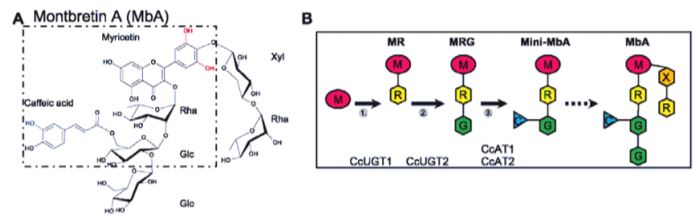
In this issue of Plant Physiology, Irmisch et al. (2019) investigated the potential of Nicotiana benthamiana as a heterologous plant host for production of the plant metabolite montbretin A (MbA) and its precursor mini-MbA (Figure 1). Both mini-MbA and MbA are glycosylated flavonoids that inhibit human pancreatic a-amylase, which is a promising drug target for treatment of type-2 diabetes, and they can control blood sugar levels in an animal model. MbA is found in underground root organs, called corms, of montbretia (Crocosmia x crocosmiiflora), but concentrations are low and do not permit isolation of enough material for drug trials.
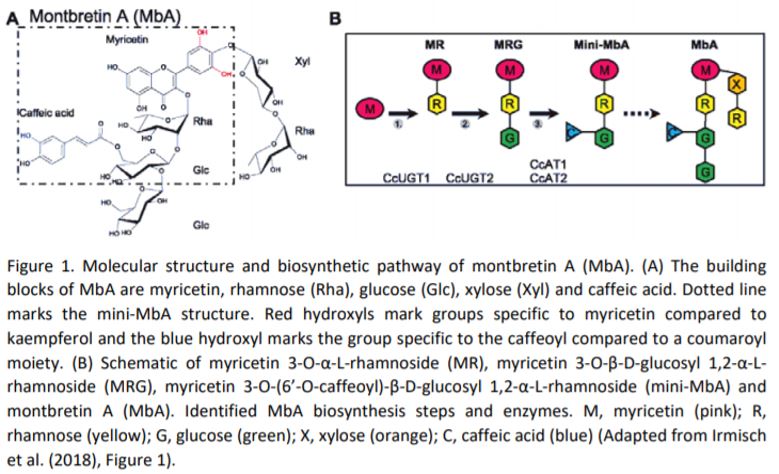 Enzymes that catalyze the formation of the first two pathway-specific intermediates in MbA biosynthesis were previously characterized using the same N. benthamiana expression system (Figure 1)(Irmisch et al., 2018), but mini-MbA was not produced in this system, as N. benthamiana, along with most other plants, does not produce the key intermediate myricetin. The genes for myricetin production from montbretia were identified in this publication through a combination of transcriptomics of corms and biochemical characterization of the individual enzymes. Interestingly, most enzymes accepted a range of substrates; as a result, biosynthesis of myricetin is a metabolic network and not a linear pathway, and combined expression in N. benthamiana enabled production of myricetin. Addition of the previously identified enzymes largely enabled production of precursors and mini-MbB that has a coumaroyl group instead of the caffeoyl one present in mini-MbA.
Enzymes that catalyze the formation of the first two pathway-specific intermediates in MbA biosynthesis were previously characterized using the same N. benthamiana expression system (Figure 1)(Irmisch et al., 2018), but mini-MbA was not produced in this system, as N. benthamiana, along with most other plants, does not produce the key intermediate myricetin. The genes for myricetin production from montbretia were identified in this publication through a combination of transcriptomics of corms and biochemical characterization of the individual enzymes. Interestingly, most enzymes accepted a range of substrates; as a result, biosynthesis of myricetin is a metabolic network and not a linear pathway, and combined expression in N. benthamiana enabled production of myricetin. Addition of the previously identified enzymes largely enabled production of precursors and mini-MbB that has a coumaroyl group instead of the caffeoyl one present in mini-MbA.
Even the low yields for mini-MbA reported here after expression of the biosynthesis pathway in N. benthamiana could be the first step towards producing larger quantities of mini-MbA and hopefully MbA itself. However, N. benthamiana will most likely not be an ideal expression host on an industrial scale, as the Agrobacterium infiltration method is labor intensive and transient. For reliable production, a stable genome integration method is preferred. Nevertheless, this study is a wonderful example of how powerful the N. benthamiana system is for pathway discovery and optimization, as individual plasmids can be transformed individually into Agrobacterium and multiple cultures are then mixed before leaf infiltration.