Disarming the Assassins Within: Plant Cells Use S-Nitrosylation to Deactivate the HopAI1 Effectors
The assassin who injects a bead of poison by stabbing his victim with the tip of his umbrella has got nothing on plant pathogenic bacteria such as Pseudomonas syringae, which injects dozens of effector proteins into plant cells. These effectors act as tiny assassins to take out host defenses by diverse mechanisms, thus helping the pathogen to escape immunity. For example, the effector HopAI1 dephosphorylates the Mitogen-Associated Protein Kinases (MAPKs or MPKs) in the kinase cascades that activate plant defenses.
Although HopAI1 targets plant defense signaling, plant cells can still activate an immune response, including programmed cell death. Could plant cells be fighting back by inhibiting HopAI1? Nitric oxide (NO) accumulates to high levels following pathogen recognition and mediates S-nitrosylation, a protein modification where a NO group is covalently bound to cysteine. S-nitrosylation alters protein activity, localization, and protein–protein interactions and has important roles in plant responses to biotic and abiotic stress (reviewed in Romero-Puertas et al., 2013).
Work in animal systems showed that S-nitrosylation can inactivate pathogen virulence factors and Ling, Bellin, et al. (2017) hypothesized that S-nitrosylation might affect HopAI1. HopAI1 has a single, conserved cysteine and, using a biotin switch assay (which switches in a biotin molecule in place of the nitroso group) the authors showed that HopAI1 can be S-nitrosylated by treatment with S-nitrosoglutathione. They found that S-nitrosoglutathione and other NO donors inhibited the phosphothreonine lyase activity of HopAI1, but did not inhibit a cysteine-to-serine mutant (HopA1CS). The affected cysteine is not part of the HopAI1 catalytic site, so the mechanism by which S-nitrosylation affects HopAI1 enzymatic activity remains to be revealed.
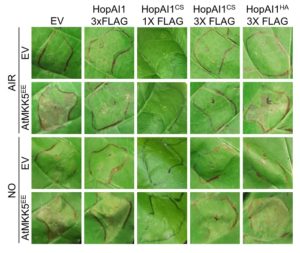 The authors used multiple assays to show that NO can inhibit HopAI1 activity in plant cells. Constitutively active forms of MKK4 induce cell death in planta and HopAI1 can suppress this cell death; the authors used this assay to show that NO inhibited HopAI1 activity in plants transiently expressing the proteins (figure). They also tested the interaction of P. syringae with Arabidopsis thaliana. To this end, they expressed HopAI1 in stable transgenic plants, or in infecting bacteria, and induced plant defenses using an effector–resistance protein interaction (AvrRpt2–RPS2 and AvrB–RPM1), or by treatment with a bacterial peptide (the flg22 fragment of flagellin). HopAI1CS produced less cell death compared with HopAI1, consistent with nitrosylation preventing HopAI1 from suppressing plant defenses. Moreover, HopAI1 and HopAI1CS behaved similarly in Arabidopsis mutants that produce less NO during the defense response. The biotin switch assay also detected S-nitrosylation of HopAI1 (but not HopAI1CS) in planta during infection. Examination of the downstream kinases and expression of MAPK-dependent genes showed that NO inhibits the effect of HopAI1 on MAPK phosphorylation and expression of downstream genes in the plant defense response.
The authors used multiple assays to show that NO can inhibit HopAI1 activity in plant cells. Constitutively active forms of MKK4 induce cell death in planta and HopAI1 can suppress this cell death; the authors used this assay to show that NO inhibited HopAI1 activity in plants transiently expressing the proteins (figure). They also tested the interaction of P. syringae with Arabidopsis thaliana. To this end, they expressed HopAI1 in stable transgenic plants, or in infecting bacteria, and induced plant defenses using an effector–resistance protein interaction (AvrRpt2–RPS2 and AvrB–RPM1), or by treatment with a bacterial peptide (the flg22 fragment of flagellin). HopAI1CS produced less cell death compared with HopAI1, consistent with nitrosylation preventing HopAI1 from suppressing plant defenses. Moreover, HopAI1 and HopAI1CS behaved similarly in Arabidopsis mutants that produce less NO during the defense response. The biotin switch assay also detected S-nitrosylation of HopAI1 (but not HopAI1CS) in planta during infection. Examination of the downstream kinases and expression of MAPK-dependent genes showed that NO inhibits the effect of HopAI1 on MAPK phosphorylation and expression of downstream genes in the plant defense response.
To recap briefly, in a scenario complex enough for any thriller (but only middling complex for plant biology), pathogen recognition causes production of NO; NO inhibits HopAI1 activity; if not reined in by NO, HopAI1’s phosphothreonine lyase activity removes MAPK phosphorylation; dephosphorylated MAPKs fail to induce the expression of downstream defense genes and defense-related cell death.
Now, what would a thriller be without a plot twist? The authors found that, compared with wild type and plants expressing HopAI1, the plants expressing HopAI1CS showed increased resistance to P. syringae carrying the effector AvrRpt2, which should induce a robust defense response. This resistance, measured as decreased bacterial growth, seems to contradict the above scenario where HopAI1CS, unhampered by S-nitrosylation, can impair host defenses. What was going on? The authors found that another defense pathway, acting through the SUMM2 protein, sensed the inhibition of one of the MAPKs, a phenomenon known as “guarding”. The SUMM2-dependent pathway then activated other defense genes to increase resistance. This shows that, when you are the target of a million tiny assassins, it helps to have more than one trick up your leaves.
REFERENCES
Bellin, D., Asai, S., Delledonne, M., and Yoshioka, H. (2013). Nitric oxide as a mediator for defense responses. Mol. Plant Microbe Interact. 26: 271-277.
Ling, T., Bellin, D., Vandelle, E., Imanifard, Z., Delledonne, M. (2017) Host-mediated S-Nitrosylation Disarms the Bacterial Effector HopAI1 to Re-establish Immunity. Plant Cell 10.1105/tpc.16.00557.
Romero-Puertas M.C., Rodríguez-Serrano M., Sandalio L.M. (2013) Protein S-nitrosylation in plants under abiotic stress: an overview. Front Plant Sci. 2013 Sep 20;4:373. doi: 10.3389/fpls.2013.00373.
Trapet, P., Kulik, A., Lamotte, O., Jeandroz, S., Bourque, S., Nicolas-Frances, V., Rosnoblet, C., Besson-Bard, A., and Wendehenne, D. (2015). NO signaling in plant immunity: A tale of messengers. Phytochemistry 112: 72-79.