Direct conversion of carlactonoic acid to orobanchol by cytochrome P450 CYP722C in strigolactone biosynthesis
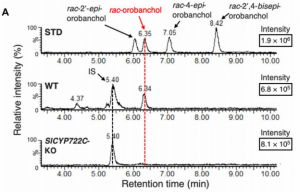
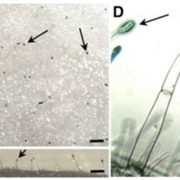
Strigolactones (SL) are a compounds that play important roles as phytohormones and as a rhizosphere signaling. Despite the great advances in their research that occurred recently, their biosynthetic pathway is still not well understood. Until now, the pathway leading to the precursor carlactonic acid (CLA) was described, where MAX1 is responsible for the last step. In this work, Takatoshi et al. identified a previously uncharacterized cytochrome P450 enzyme (CYP722C) involved in the direct biosynthesis of the canonical SL orobanchol and solanacol. This enzyme can convert CLA in orobanchol without the intermediate 4-Deoxy orobanchol as some MAX1 homologs do. They support their findings using in vitro enzyme assays and also produced a CRISPR knockout in tomato. Mutant plants did not present phenotypic defects in plant development and probably CYP722C does not explain most of SL biosynthesis. Despite that, authors provide evidence of the relevance of these compounds in rhizosphere signaling, showing impaired parasitic seed germination that could be of biotechnological interest. This article provides advances about the biosynthesis of this complex array of phytohormones, lots of exciting questions still awaits to be answered. (Summary by Facundo Romani) Science Advances 10.1126/sciadv.aax9067