Cellulose Synthase Stoichiometry Varies among Species and Tissues
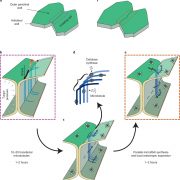
Cellulose, the most abundant biopolymer on earth, is an important structural component of the primary and secondary cell wall of plant cells. It is also found in animals (tunicates), oomycetes, and bacteria (Kumar and Turner, 2015). Besides providing support and rigidity in living organisms, cellulose has significant economic value, as it is commonly used for paper and fiber production, and cellulosic ethanol has become a promising renewable biofuel. Cellulose is produced as a cable-like structure called cellulose microfibrils (CMF), consisting of a bundle of linear chains of β-1,4-glucan. These linear chains are aligned in parallel and form crystalline structures by hydrogen bonds and Van der Waals forces, and the number of these interchain interactions affects the rigidity and accessibility for degradation of CMF (Somerville, 2006; Hill et al., 2014). CMFs are synthesized on the plasma membrane by the cellulose synthase complex (CSC), which forms a hexameric rosette structure that can be visualized by freeze fracture transmission electron microscopy (Kimura et al., 1999). Understanding the structure of the CSC is prerequisite for engineering cellulose production for biofuel purposes.
Numerous genetic and biochemical analyses have been used to resolve the composition and stoichiometry of the CSC and have generally concluded that three different cellulose synthases (CesAs) are required to form a functional CSC. In Arabidopsis (Arabidopsis thaliana), CesA1, CesA3, and CesA6-like (including CesA2, CesA5, CesA6, and CesA9) are involved in primary cell wall biosynthesis, and CesA4, CesA7, and CesA8 form the CSC in secondary cell wall biosynthesis (Somerville, 2006; Kumar and Turner, 2015). However, the number, arrangement, and stoichiometry of the CesA units remain controversial (Somerville, 2006; Gonneau et al., 2014; Hill et al., 2014). Recent studies using 3-d-old dark-grown Arabidopsis seedlings and 6- to 7-week-old Arabidopsis stems have shown equimolar amounts of CesA proteins in both the CSC of primary cell walls (Gonneau et al., 2014) and that of secondary cell walls (Hill et al., 2014), making 1:1:1 stoichiometry the most likely CSC model.
In this issue of Plant Physiology, Zhang et al. (2018) apply quantitative proteomics to quantify the stoichiometry of CesAs in the dicotyledonous angiosperms Arabidopsis and aspen (Populus tremula) and a gymnosperm, Norway spruce (Picea abies), and find that the stoichiometry of CesA proteins can be species and tissue specific. The authors develop a method of rapidly extracting proteins from differentiating xylem of the three species, followed by mass spectrometry and peptide quantification using multiple reaction monitoring. They validate the 1:1:1 stoichiometry of CesA4, CesA7, and CesA8 in 6-week-old Arabidopsis stems, as well as in differentiating xylem of Norway spruce. Surprisingly, the stoichiometry of CesA4, CesA7a/b, and CesA8a/b is 2:1:3 in differentiating xylem of aspen and 3:1:8 in tension wood, suggesting alternatives to the equimolar stoichiometry model in certain species and tissue types. Moreover, cell wall structure in the tension wood of aspen is also different from that of many other members of the Salicaceae sensu lato (Ghislain et al., 2016), further supporting the variation of cell wall structures in different plant species.
The thickness of CMF depends on the number of glucan chains included, which correlates with the number and stoichiometry of catalytically active CesAs in the CSC. However, the 2:1:3 stoichiometry in aspen xylem does not result in a difference in the crystalline CMF diameter compared to the xylem of spruce (Zhang et al., 2018). The tension wood of aspen does have thicker CMF, but a 3:1:8 stoichiometry would appear to require 12 CesAs in each lobe of the CSC rosette, which is too big and also inconsistent with other data (Somerville, 2006; Kumar and Turner, 2015). The authors did point out the possibility of isolated CesA isoforms that are not in the CSC, so that the ratio may not accurately reflect the stoichiometry in the CSC itself (Zhang et al., 2018). Alternatively, some of the CesA proteins may not always function in heterotrimers. Both heterologously expressed CesA8 from a hybrid aspen and CesA5 from moss (Physcomitrella patens) are able to produce CMF in the absence of their CesA partners in vitro (Purushotham et al., 2016; Cho et al., 2017). Although CesA2, CesA5, and CesA9 are all involved in cellulose production in the Arabidopsis seed coat, only CesA5 but not CesA2 or CesA9 is required for mucilage biosynthesis (Mendu et al., 2011; Kumar and Turner, 2015), implicating unique functions of certain CesA members.
So far, the 1:1:1 stoichiometry with three CesAs in each lobe of the CSC rosette and 18 CesAs in total producing 18 glucan chains is the most widely accepted CSC model. The study of Zhang et al. (2018) suggests that the CSC may have modifications in different species and tissue types, an observation that will be informative in transferring the knowledge from model species into bioenergy crops.
REFERENCES
Cho SH, Purushotham P, Fang C, Maranas C, Díaz-Moreno SM, Bulone V, Zimmer J, Kumar M, Nixon BT (2017) Synthesis and self-assembly of cellulose microfibrils from reconstituted cellulose synthase. Plant Physiol 175: 146–156
Ghislain B, Nicolini E-A, Romain R, Ruelle J, Yoshinaga A, Alford MH, Clair B (2016) Multilayered structure of tension wood cell walls in Salicaceae sensu lato and its taxonomic significance. Bot J Linn Soc 182: 744–756
Gonneau M, Desprez T, Guillot A, Vernhettes S, Höfte H (2014) Catalytic subunit stoichiometry within the cellulose synthase complex. Plant Physiol 166: 1709–1712
Hill JL Jr., Hammudi MB, Tien M (2014) The Arabidopsis cellulose synthase complex: a proposed hexamer of CESA trimers in an equimolar stoichiometry. Plant Cell 26: 4834–4842
Kimura S, Laosinchai W, Itoh T, Cui X, Linder CR, Brown RM Jr. (1999) Immunogold labeling of rosette terminal cellulose-synthesizing complexes in the vascular plant Vigna angularis. Plant Cell 11: 2075–2086
Kumar M, Turner S (2015) Plant cellulose synthesis: CESA proteins crossing kingdoms. Phytochemistry 112: 91–99
Mendu V, Griffiths JS, Persson S, Stork J, Downie AB, Voiniciuc C, Haughn GW, DeBolt S (2011) Subfunctionalization of cellulose synthases in seed coat epidermal cells mediates secondary radial wall synthesis and mucilage attachment. Plant Physiol 157: 441–453
Purushotham P, Cho SH, Díaz-Moreno SM, Kumar M, Nixon BT, Bulone V, Zimmer J (2016) A single heterologously expressed plant cellulose synthase isoform is sufficient for cellulose microfibril formation in vitro. Proc Natl Acad Sci USA 113: 11360–11365
Somerville C (2006) Cellulose synthesis in higher plants. Annu Rev Cell Dev Biol 22: 53–78