Cell Wall Polymers: The Importance of Deacetylation
Many of the polysaccharides that make up the plant cell wall carry acetate side groups. Notably, the degree of such acetylation is not always the same—even within the same species, it changes over the course of development and is different between tissues. While the mechanism for adding acetyl groups to carbohydrates is generally conserved between kingdoms (reviewed in Gille and Pauly, 2012), the biological function of cell wall polymer acetylation is still not entirely clear. In addition, recent work highlights the importance of not just the presence of acetylation in the cell wall, but also the removal acetyl groups. Initially, Zhang et al. (2017) found that the rice (Oryza sativa) brittle leaf sheath1 (bs1) mutant was defective in a GDSL esterase and showed that this esterase deacetylates the hemicellulose xylan. Now, Zhang et al. (2019) have characterized DEACETYLASE ON THE ARABINOSYL SIDECHAIN OF XYLAN 1 (DARX1), which deacetylates arabinose residues of arabinoxylan, with consequences for the structure of the rice cell wall.
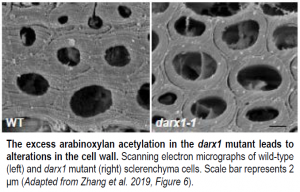 Arabinoxylan is abundant in the cell wall and is often acetylated. Zhang and coauthors focused on elucidating the factors that generate the pattern of acetylation in arabinoxylan, identifying DARX1 in an enzymatic screen for rice glycosyl deacetylases that act on xylose and arabinose residues. The recombinant DARX1 protein displayed esterase activity, and darx1 null mutant plants had higher levels of arabinoxylan acetylation in the cell wall compared to the wild type. The authors traced this difference to an acetyl group in the mutant at an arabinosyl reside that is not acetylated in wild type. Further analysis demonstrated that DARX1 has deacetylase activity with preference for specific sites within arabinoxylan.
Arabinoxylan is abundant in the cell wall and is often acetylated. Zhang and coauthors focused on elucidating the factors that generate the pattern of acetylation in arabinoxylan, identifying DARX1 in an enzymatic screen for rice glycosyl deacetylases that act on xylose and arabinose residues. The recombinant DARX1 protein displayed esterase activity, and darx1 null mutant plants had higher levels of arabinoxylan acetylation in the cell wall compared to the wild type. The authors traced this difference to an acetyl group in the mutant at an arabinosyl reside that is not acetylated in wild type. Further analysis demonstrated that DARX1 has deacetylase activity with preference for specific sites within arabinoxylan.
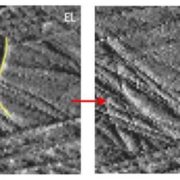
The physiological importance of this deacetylation is underscored by the consequences of the increased arabinoxylan acetylation in the darx1 mutant. The mutant had aberrant interactions between cellulose and xylan in the cell wall, as well as altered cellulose microfibril orientation. The cell walls themselves were thinner in the mutant than in the wild type, with less cellulose (see Figure). These walls were weaker, which led to morphological alterations in the mutant plants.
Zhang et al. went on to show that DARX1 is present in the Golgi. Thus, it appears that specific deacetylation takes place as the cell wall polymers are produced in the Golgi, rather than after they are delivered to the apoplast for integration into the cell wall. Together, these results highlight the complexity of wall polymer synthesis—and its potential for plasticity.
REFERENCES
Gille, S., and Pauly, M. (2012). O-acetylation of plant cell wall polysaccharides. Front. Plant Sci. 3: 12.
Zhang, B., Zhang, L., Li, F., Zhang, D., Liu, X., Wang, H., Xu, Z., Chu, C., and Zhou, Y. (2017). Control of secondary cell wall patterning involves xylan deacetylation by a GDSL esterase. Nat. Plants 3: 17017.
Zhang, L., Gao, C., Mentink-Vigier, F., Tang, L., Zhang, D., Shaogan Wang, S., Cao, S., Xu, Z., Liu, X., Wang, T., Zhou, Y., Zhang, B. (2019). Arabinosyl Deacetylase Modulates the Arabinoxylan Acetylation Profile and Secondary Wall Formation. Plant Cell. https://doi: 10.1105/tpc.18.00849.