Balance Under Stress: A Case of Mixed Signals
Plants may appear to be sitting ducks to whatever pest, pathogen or bad weather assaults them, but they are far from defenseless. In fact, plants are artful masters of war, armed with elaborate physical and chemical defense systems. But, in many cases, optimizing growth and defense is like running a business or a laboratory on a budget. A plant has a certain amount of resources that may be expended on the production of vegetative and reproductive tissues as well as physical barriers and defense compounds (Züst & Agrawal, 2017). To successfully manage all these activities, plants must acquire adequate resources to fund them.
Both excess or shortage of resources, such as potassium (K) or nitrogen (N), can lead to the overproduction of reactive oxygen species (ROS) (Shin et al., 2005, Shin & Schachtman, 2004), which wreak havoc on cellular membranes, proteins and DNA (Moller et al., 2007). To minimize the damage from and, if possible, escape the source, plants must properly perceive, diagnose and respond to the stress. As we rely on plants for myriad essential resources including food, medicine, fuel, soil stabilization, shelter and clothing, unraveling the intricate processes plants use to perceive and respond to various stresses may help us optimize crop yield under stressful conditions.
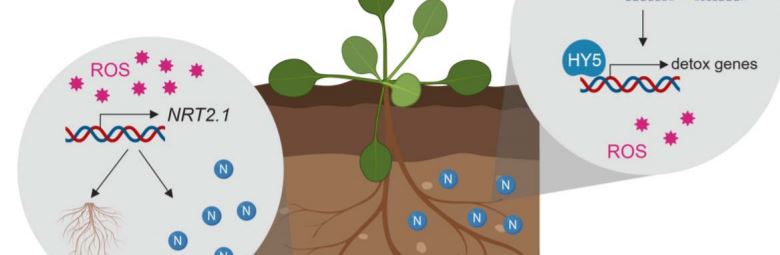
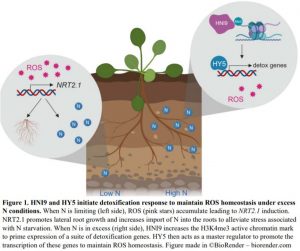 Plant mutants are useful tools for discovering key elements of stress response pathways. In this issue of Plant Physiology, Bellegarde et al. (2019) investigate the role of a modifier protein called HIGH NITROGEN INSENSITIVE 9 (HNI9) in alleviating stress caused by excess N (Bellegarde et al., 2019). They started with the observation that hni9-1 mutant plants have increased expression of the nitrate transporter NRT2.1 when N is abundant. In wild-type Arabidopsis thaliana plants, NRT2.1 is upregulated in response to N starvation to promote the production of lateral roots to hunt down the limiting nutrient and to actively import N into the roots (Remans et al., 2006) (Figure 1). Inducing NRT2.1 under excess N conditions is the exact opposite of the desired response, revealing hni9-1 mutant plants somehow have their signals crossed. So, what exactly is the connection between NRT2.1 and HNI9?
Plant mutants are useful tools for discovering key elements of stress response pathways. In this issue of Plant Physiology, Bellegarde et al. (2019) investigate the role of a modifier protein called HIGH NITROGEN INSENSITIVE 9 (HNI9) in alleviating stress caused by excess N (Bellegarde et al., 2019). They started with the observation that hni9-1 mutant plants have increased expression of the nitrate transporter NRT2.1 when N is abundant. In wild-type Arabidopsis thaliana plants, NRT2.1 is upregulated in response to N starvation to promote the production of lateral roots to hunt down the limiting nutrient and to actively import N into the roots (Remans et al., 2006) (Figure 1). Inducing NRT2.1 under excess N conditions is the exact opposite of the desired response, revealing hni9-1 mutant plants somehow have their signals crossed. So, what exactly is the connection between NRT2.1 and HNI9?
HNI9 is a modifying factor of chromatin, which is a combination of DNA and its protein packaging. Just like football coaches use images or signals to call plays from the sideline, chromatin modifiers use specific marks to make genes open or closed for expression. Bellegarde et al. show that, in response to excess N, HNI9 tags a suite of detoxification genes with a specific active chromatin mark. Then, the transcription factor, HY5, acts as the quarterback, or master regulator, to ensure these genes are expressed. As a result, ROS homeostasis is maintained thereby preventing the mis-regulation of NRT2.1 (Figure 1). Since HY5 induces NRT2.1 expression in the roots under other circumstances (Chen et al., 2016), it is critical that HNI9 properly mark the DNA so HY5 activates the appropriate set of genes. If either HNI9 or HY5 are inactivated, under excess N conditions, the right DNA marks are not made or there is no regulator to act on them. This leads to ROS overaccumulation, NRT2.1 induction and stunted primary root growth.
Bellegarde et al. also show that ROS generating compounds induce NRT2.1 in wild-type plants, indicating that both loss of HNI9 negative regulation (Widiez et al., 2011) and ROS overaccumulation may contribute to NRT2.1 induction under high N conditions in hni9-1 mutants. As elevated ROS occurs in wild type plants under N starvation (Shin et al., 2005), this may be an important trigger to appropriately induce NRT2.1 to deal with that stress.
Depending on the stress severity, the potential outcomes for incorrect responses to stressful situations are deficiencies in growth, development and reproduction or possibly plant death. By identifying key players involved in maintaining ROS homeostasis in response to excess N, Bellegarde et al. have deepened our understanding of one of the plant’s many complex stress response pathways, moving us one step closer to our goal of maximizing plant growth and yield under sub-optimal conditions.
References:
Bellegarde F, Maghiaoui A, Boucherez J, et al., 2019. The Chromatin Factor HNI9 and ELONGATED HYPOCOTYL 5 Maintain ROS Homeostasis under High Nitrogen Provision. Plant Physiology, pp.01473.2018.
Chen X, Yao Q, Gao X, Jiang C, Harberd NP, Fu X, 2016. Shoot-to-Root Mobile Transcription Factor HY5 Coordinates Plant Carbon and Nitrogen Acquisition. Curr Biol 26, 640-6.
Moller IM, Jensen PE, Hansson A, 2007. Oxidative modifications to cellular components in plants. Annu Rev Plant Biol 58, 459-81.
Remans T, Nacry P, Pervent M, et al., 2006. A central role for the nitrate transporter NRT2.1 in the integrated morphological and physiological responses of the root system to nitrogen limitation in Arabidopsis. Plant Physiology 140, 909-21.
Shin R, Berg RH, Schachtman DP, 2005. Reactive oxygen species and root hairs in Arabidopsis root response to nitrogen, phosphorus and potassium deficiency. Plant Cell Physiol 46, 1350-7.
Shin R, Schachtman DP, 2004. Hydrogen peroxide mediates plant root cell response to nutrient deprivation. Proc Natl Acad Sci U S A 101, 8827-32.
Widiez T, El Kafafi ES, Girin T, et al., 2011. HIGH NITROGEN INSENSITIVE 9 (HNI9)-mediated systemic repression of root NO-3 uptake is associated with changes in histone methylation. Proceedings of the National Academy of Sciences 108, 13329.
Züst T, Agrawal AA, 2017. Trade-Offs Between Plant Growth and Defense Against Insect Herbivory: An Emerging Mechanistic Synthesis. In. Annu Rev Plant Biol. 513-34. (68.)
Figure legend:
Camalexin-centered Biochemical Defense Provides Resistance to Fungal Invasion. A simplified schematic illustrates the Arabidopsis camalexin response (adapted from Figs 10 & 11 in He et al., 2019). Botrytis cinerea (Bc) infection detected and transduced through MAP kinase signalling cascades activates WRKY33, and in turn, upregulates camalexin biosynthesis (CYP71A13 & PAD3) and transport (PEN3 & PDR12) genes. PEN3 and PDR12 redundantly transport camalexin and (likely) additional unknown trp-derived metabolites to suppresses fungal growth. The specific role of PEN3 in transporting indole-glucosinolates (IGs) does not contribute to resistance against B. cinerea.