An Intrinsically Disordered Protein Interacts with the Plant Cytoskeleton
Intrinsically disordered proteins lack a defined three-dimensional structure but often contain a simple amino acid composition with repeated sequences that provide the basis for multivalent intermolecular interactions. Because of their unique structural flexibility, 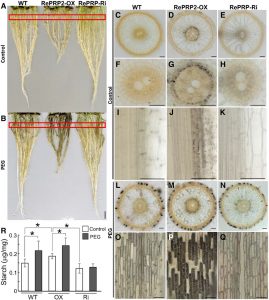 conformational adaptability, and ability to react quickly in response to changing environments, intrinsically disordered proteins often function as hubs of protein-protein interaction networks in response to environmental stresses. For example, tardigrade disordered proteins are essential for their high level of desiccation tolerance, and disordered LATE EMBRYOGENESIS ABUNDANT (LEA) proteins often accumulate under dehydration and osmotic stresses in plants. Hsiao et al. (10.1104/pp.19.01372) now shed light on the role of an intrinsically disordered protein, REPETITIVE PRO-RICH PROTEIN (RePRP), in regulating rice root growth under water deficit. Containing nearly 40% Pro, RePRP is induced by water deficit and abscisic acid (ABA) in the root elongation zone. RePRP is both sufficient and necessary for repression of root development by water deficit or ABA. The authors show that RePRP interacts with actin and tubulin both in vivo and in vitro. Binding of RePRP reduces the abundance of actin filaments, thus diminishing noncellulosic polysaccharide transport to the cell wall and increasing the enzyme activity of Suc synthase. RePRP also reorients the microtubule network, which leads to disordered cellulose microfibril organization in the cell wall and suppresses root cell elongation., thereby promote more starch accumulation in the “short-but-heavy” roots. These results demonstrate a novel role of an ABA/stress-induced intrinsically disordered protein in regulating plant root growth under water deficit.
conformational adaptability, and ability to react quickly in response to changing environments, intrinsically disordered proteins often function as hubs of protein-protein interaction networks in response to environmental stresses. For example, tardigrade disordered proteins are essential for their high level of desiccation tolerance, and disordered LATE EMBRYOGENESIS ABUNDANT (LEA) proteins often accumulate under dehydration and osmotic stresses in plants. Hsiao et al. (10.1104/pp.19.01372) now shed light on the role of an intrinsically disordered protein, REPETITIVE PRO-RICH PROTEIN (RePRP), in regulating rice root growth under water deficit. Containing nearly 40% Pro, RePRP is induced by water deficit and abscisic acid (ABA) in the root elongation zone. RePRP is both sufficient and necessary for repression of root development by water deficit or ABA. The authors show that RePRP interacts with actin and tubulin both in vivo and in vitro. Binding of RePRP reduces the abundance of actin filaments, thus diminishing noncellulosic polysaccharide transport to the cell wall and increasing the enzyme activity of Suc synthase. RePRP also reorients the microtubule network, which leads to disordered cellulose microfibril organization in the cell wall and suppresses root cell elongation., thereby promote more starch accumulation in the “short-but-heavy” roots. These results demonstrate a novel role of an ABA/stress-induced intrinsically disordered protein in regulating plant root growth under water deficit.