Adjusting Boron Transport by Two-Step Tuning of Levels of the Efflux Transporter BOR1
Boron is an essential plant micronutrient with the narrowest optimal range in the soil of any micronutrient. At neutral pH, boron is present as uncharged boric acid, B(OH)3, which can freely penetrate membranes. Boron plays an important role in cross-linking cell wall components, but boron starvation and toxicity affect various metabolic and physiological processes beyond cell wall reinforcement (Reid, 2010). Controlling boron transport is therefore in the best interest of developing plants.
Under boron-starvation conditions, boron uptake and mobilization toward the shoot is facilitated by the transporters NIP5;1 and BOR1. NIP5;1 is only expressed in the outer root layer, enhancing permeability of boron and passive boron uptake from the soil (Takano et al., 2010). BOR1 is a boron efflux transporter expressed in multiple cell layers of the root. In the endodermis, BOR1 polar localization toward the vasculature ensures efficient boron mobilization to the shoot (Takano et al., 2010). Under boron-sufficient conditions, BOR1 undergoes ubiquitination and degradation, reducing boron concentrations in the shoot (Kasai et al., 2011). However, intracellular boron levels decreased under boron-toxic conditions, even in lines expressing BOR1 with a mutated ubiquitination site (K590A), suggesting the existence of another mechanism controlling BOR1 levels.
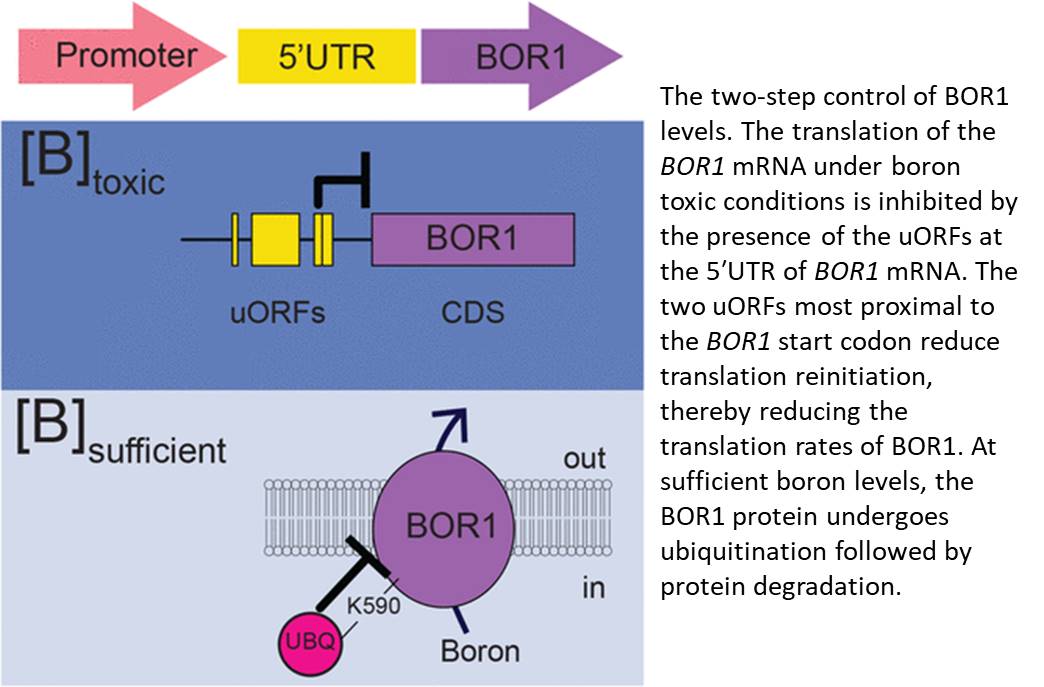
Now, an article by Aibara et al. (2018) published in the current issue of Plant Physiology describes another mechanism of BOR1 regulation by its own 5′ untranslated region (UTR). The authors identified four untranslated open reading frames (uORFs) in the 5′UTR of the BOR1 mRNA. Using reporter gene constructs containing a truncated BOR1 5′UTR and with the start codon AUG replaced with AAG for each uORF, the authors found that the two uORFs closest to the start codon of BOR1 have a role in regulating boron-dependent activity of the reporter gene. The boron-dependent effect of the 5′UTR on reporter gene activity was also observed in vitro using wheat germ extract. These results suggested that high levels of boron might directly interfere with the translation of BOR1. The two uORFs closest to the start codon affected the rate of translational reinitiation, as shortening the distance between the last uORF and the main ORF abolished boron-dependent activity of the reporter gene.
One potential mechanism underlying this effect on reinitiation of translation is that toxic levels of boric acid might reduce formation of ternary complexes (TCs), which are necessary for reinitiating translation. TCs are composed of GTP-bound eukaryotic Initiation Factor 2 (eIF2) and Met-bound tRNA. In yeast, toxic boron conditions enhanced phosphorylation of the α-subunit of eIF2, reducing the formation of TCs (Uluisik et al., 2011). Under boron-toxic conditions, presumably when the levels of TC are low, the distance between the last two uORFs and the BOR1 start codon prevent the reassembly of the translational machinery and reduce translation of BOR1. This mechanism of boron-dependent translational repression is, thus far, unique to BOR1 and possibly to its orthologs in other species. Other boron transporters regulated by 5′UTRs include NIP5;1, but the mechanisms regulating these transporters rely on ribosome stalling at the uORF (Tanaka et al., 2016), which did not occur in the 5′UTR of BOR1.
The described 5′UTR uORF-mediated mechanism of decreased BOR1 translation occurs at toxic levels of boron, indicating a stepwise control of boron transport. Boron accumulation varies along the longitudinal root axis (Shimotohno et al., 2015), and levels of BOR1 are reduced in the exodermis, but not in the endodermis, of rice (Oryza sativa) roots grown under boron-sufficient conditions, compared with roots grown under boron starvation (Nakagawa et al., 2007). The proposed two-step control of BOR1 levels (Fig. 1) could operate simultaneously in different cell types varying in boron accumulation. Implementing this two-step control mechanism in models will help to explain cell type-specific regulation of boron transport under boron-sufficient and toxic conditions.
In the natural environment, boron toxicity co-occurs with salt stress (Reid, 2010). Interestingly, high levels of boron can alleviate the symptoms of salt stress by promoting the recovery of cell wall stiffness, as recently described for the feronia mutant (Feng et al., 2018). Evaluation of BOR1 activity in the context of boron and salinity stress would be an important contribution for plant breeding efforts for regions where those two stresses limit plant productivity.
Additionally, the exploration of the two-step mechanism of BOR1 control could shed light on boron sensitivity of agronomically important plants. Overexpression of BOR1(K590A), which exhibits weaker polar localization in the endodermis, enhanced tolerance to high boron concentrations (Wakuta et al., 2016). The BOR1-like 5′UTR uORFs limiting the translation at high boron concentrations present in boron transporters with nonpolar localization could unveil factors limiting boron tolerance. The discovery of this two-step regulation of boron transporter reveals not only an elegant mechanism of boron transport control that depends on boron levels, but also unleashes new possibilities for strategies to breed for enhanced boron tolerance.
REFERENCES
Aibara I, Hirai T, Kasai K, Takano J, Onouchi H, Naito S, Fujiwara T, Miwa K (2018) Boron-dependent translational suppression of a borate exporter BOR1 for avoidance of boron toxicity. Plant Physiol. 177: 759–774
Feng W, Kita D, Peaucelle A, Cartwright HN, Doan V, Duan Q, Liu M-C, Maman J, Steinhorst L, Schmitz-Thom I, et al. (2018) The FERONIA receptor kinase maintains cell-wall integrity during salt stress through Ca2+ signaling. Curr Biol 28: 666–675
Kasai K, Takano J, Miwa K, Toyoda A, Fujiwara T (2011) High boron-induced ubiquitination regulates vacuolar sorting of the BOR1 borate transporter in Arabidopsis thaliana. J Biol Chem 286: 6175–6183
Nakagawa Y, Hanaoka H, Kobayashi M, Miyoshi K, Miwa K, Fujiwara T (2007) Cell-type specificity of the expression of Os BOR1, a rice efflux boron transporter gene, is regulated in response to boron availability for efficient boron uptake and xylem loading. Plant Cell 19: 2624–2635
Reid R (2010) Can we really increase yields by making crop plants tolerant to boron toxicity? Plant Sci 178: 9–11
Shimotohno A, Sotta N, Sato T, De Ruvo M, Marée AFM, Grieneisen VA, Fujiwara T (2015) Mathematical modeling and experimental validation of the spatial distribution of boron in the root of Arabidopsis thaliana identify high boron accumulation in the tip and predict a distinct root tip uptake function. Plant Cell Physiol 56: 620–630
Takano J, Tanaka M, Toyoda A, Miwa K, Kasai K, Fuji K, Onouchi H, Naito S, Fujiwara T (2010) Polar localization and degradation of Arabidopsis boron transporters through distinct trafficking pathways. Proc Natl Acad Sci USA 107: 5220–5225
Tanaka M, Sotta N, Yamazumi Y, Yamashita Y, Miwa K, Murota K, Chiba Y, Hirai MY, Akiyama T, Onouchi H, Naito S, Fujiwara T (2016) The minimum open reading frame, AUG-Stop, induces boron-dependent ribosome stalling and mRNA degradation. Plant Cell 28: 2830–2849
Uluisik I, Kaya A, Fomenko DE, Karakaya HC, Carlson BA, Gladyshev VN, Koc A (2011) Boron stress activates the general amino acid control mechanism and inhibits protein synthesis. PLoS One 6: e27772
Wakuta S, Fujikawa T, Naito S, Takano J (2016) Tolerance to excess-boron conditions acquired by stabilization of a BOR1 variant with weak polarity in Arabidopsis. Front Cell Dev Biol 4: 4