A ubiquitinated SNARE protein functions in nutrient stress responses
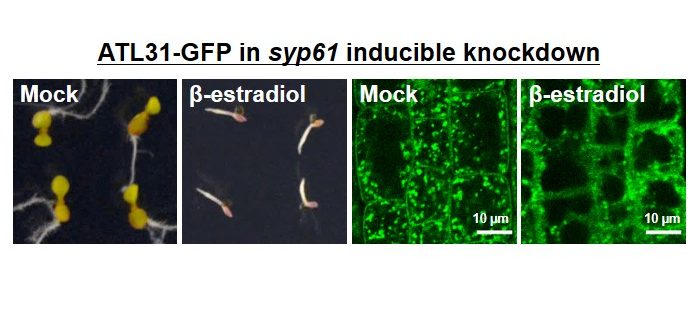
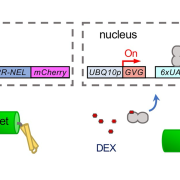
Background: Ubiquitination is a post-translational modification that functions in numerous cellular processes by serving as a molecular signal, such as protein degradation, membrane trafficking, and complex formation. Although numerous reports describe the ubiquitination of cargo proteins, the ubiquitination of components of the trafficking machinery is not well understood. We previously identified the transmembrane ubiquitin ligase ATL31 and demonstrated that it regulates carbon (C)/nitrogen (N)-nutrient stress responses in Arabidopsis. As ATL31 requires its transmembrane domain for this function, we characterized the subcellular localization of ATL3 and determined that it interacts with the TGN/EE-localized SNARE protein SYP61.
Question: SNARE proteins are key regulators of membrane trafficking. However, their roles in plant nutrient responses were not well known. Also, the ubiquitination of SNARE proteins was not well characterized. Therefore, we investigated whether the SNARE protein SYP61 is involved in plant C/N-nutrient responses and is ubiquitinated.
Findings: We found that SYP61 is required for the proper subcellular localization of ATL31 and its function in high C/low N-nutrient stress responses. In addition, we discovered that SYP61 is transiently ubiquitinated in response to low C/high N-nutrient conditions. Our results demonstrate the importance of membrane trafficking regulation in plant nutrient responses, and they suggest that SNARE proteins might be regulated by ubiquitination in response to nutrient conditions.
Next steps: We are currently trying to reveal the downstream effects of SNARE ubiquitination. It would also be interesting to investigate how common this modification is among SNARE proteins. Further experiments including analysis of ubiquitin-deficient mutants of SNARE proteins will help us understand the dynamics of the post-translational regulation of the membrane trafficking machinery.
Yoko Hasegawa, Thais Huarancca Reyes, Tomohiro Uemura, Anirban Baral, Akari Fujimaki, Yongming Luo, Yoshie Morita, Yasushi Saeki, Shugo Maekawa, Shigetaka Yasuda, Koki Mukuta, Yoichiro Fukao, Keiji Tanaka, Akihiko Nakano, Junpei Takagi, Rishikesh P. Bhalerao, Junji Yamaguchi, and Takeo Sato (2022). The TGN/EE SNARE protein SYP61 and the ubiquitin ligase ATL31 cooperatively regulate plant responses to carbon/nitrogen conditions in Arabidopsis. Plant Cell. https://doi.org/10.1093/plcell/koac014