A plant protein structure from the MATE family, an important family of transporters ($)
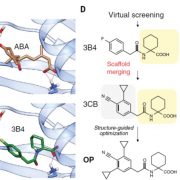
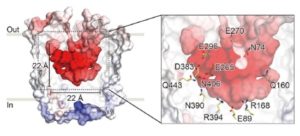 The MATE (multidrug and toxic compound extrusion) family is found in all three domains of life. Proteins in this family are secondary transporters, functioning as sodium or proton/organic cation antiporters. This family has diverse functions in plants, including vacuolar sequestration of alkaloids, regulation of local auxin biosynthesis, iron uptake and aluminum tolerance. While the crystal structures of MATE proteins from five prokaryotes have been published, Tanaka et al. have described the first plant MATE structure. This MATE (CasMATE), from Camelina sativa, was found to have two sets of six transmembrane alpha-helices and was crystallized in an outward-facing orientation. While CasMATE has a similar cation binding pocket as a known bacterial MATE, there were many differences. This CasMATE structure is an example of the binding pocket structure of plant MATE proteins, providing insight into substrate binding and specificity. (Summary by Julia Miller) Structure 10.1016/j.str.2017.07.009
The MATE (multidrug and toxic compound extrusion) family is found in all three domains of life. Proteins in this family are secondary transporters, functioning as sodium or proton/organic cation antiporters. This family has diverse functions in plants, including vacuolar sequestration of alkaloids, regulation of local auxin biosynthesis, iron uptake and aluminum tolerance. While the crystal structures of MATE proteins from five prokaryotes have been published, Tanaka et al. have described the first plant MATE structure. This MATE (CasMATE), from Camelina sativa, was found to have two sets of six transmembrane alpha-helices and was crystallized in an outward-facing orientation. While CasMATE has a similar cation binding pocket as a known bacterial MATE, there were many differences. This CasMATE structure is an example of the binding pocket structure of plant MATE proteins, providing insight into substrate binding and specificity. (Summary by Julia Miller) Structure 10.1016/j.str.2017.07.009