Xylem-mobile Oxylipins are Critical Regulators of Induced Systemic Resistance in Maize
In addition to promoting plant growth and development, the colonization of roots by beneficial microorganisms often provides aboveground tissues with enhanced resistance to pathogen attack. This form of resistance, referred to as ‘induced systemic resistance’ (ISR), relies on the long-distance movement of root-derived signals that travel through the vasculature to access and prime defenses in aerial plant organs (Pieterse et al., 2014).
The growth-promoting fungus Trichoderma virens is a potent activator of ISR in several angiosperms. In the monocot Zea mays (maize), the ability to manifest ISR relies on secreted fungal proteins that modulate host oxylipin biosynthesis (Djonović et al., 2007; Constantino et al., 2013). In particular, the 9-lipoxygenase LOX3 has emerged as a negative regulator of ISR, such that lox3 mutants exhibit constitutive resistance to foliar pathogens and xylem-sap collected from these plants is sufficient to induce ISR in wild-type receiver plants (Constantino et al., 2013).
 To further explore oxylipin-based ISR signaling, Wang et al. (2019) took an analytical approach that leveraged fungal and maize germplasm with contrasting capacities to manifest ISR. First, the authors investigated the role of the 13-lipoxygenase LOX10 during fungal colonization in maize roots. Wild-type T. virens and T. virens Δsir1 mutants (Tv–Δsir1) with enhanced ISR activity both induced LOX10 expression in maize roots, unlike ISR-deficient T. virens Δsm1 (Tv-Δsm1). To better define the role of LOX10 during ISR, the authors assessed lox10 mutants for T. virens-induced ISR to fungal infection in distant leaves. Surprisingly, lox10 mutants colonized by T. virens displayed ‘systemic-induced susceptibility’ to Colletotrichum graminicola (hemibiotroph) and Cochliobolus heterostrophus (necrotroph) compared to untreated plants. Xylem-sap swapping experiments further underscored the importance of oxylipins for ISR, as fluids collected from T. virens-treated lox10 produced systemic-induced susceptibility in wild-type receiver plants while sap collected from untreated lox3 mutants (constitutive ISR) or T. virens-treated wild-type plants increased ISR in untreated wild-type receiver plants.
To further explore oxylipin-based ISR signaling, Wang et al. (2019) took an analytical approach that leveraged fungal and maize germplasm with contrasting capacities to manifest ISR. First, the authors investigated the role of the 13-lipoxygenase LOX10 during fungal colonization in maize roots. Wild-type T. virens and T. virens Δsir1 mutants (Tv–Δsir1) with enhanced ISR activity both induced LOX10 expression in maize roots, unlike ISR-deficient T. virens Δsm1 (Tv-Δsm1). To better define the role of LOX10 during ISR, the authors assessed lox10 mutants for T. virens-induced ISR to fungal infection in distant leaves. Surprisingly, lox10 mutants colonized by T. virens displayed ‘systemic-induced susceptibility’ to Colletotrichum graminicola (hemibiotroph) and Cochliobolus heterostrophus (necrotroph) compared to untreated plants. Xylem-sap swapping experiments further underscored the importance of oxylipins for ISR, as fluids collected from T. virens-treated lox10 produced systemic-induced susceptibility in wild-type receiver plants while sap collected from untreated lox3 mutants (constitutive ISR) or T. virens-treated wild-type plants increased ISR in untreated wild-type receiver plants.
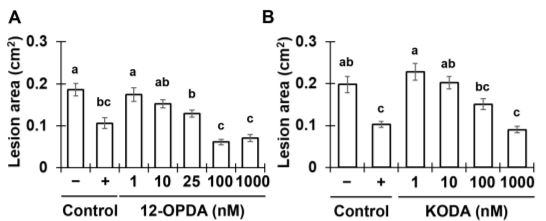
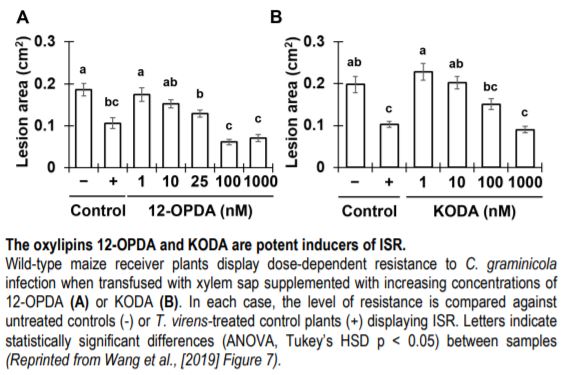
The contrasting ISR phenotypes of Tv-Δsm1/Tv-Δsir1 and maize lipoxygenase mutants facilitated comparative analytical studies aimed at identifying oxylipin ISR signals in xylem sap. To this end, the authors identified two xylem-mobile oxylipins that were strongly associated with ISR; the jasmonic acid (JA) precursor 12-oxo-phytodienoic acid (12-OPDA) and an ⍺-ketol of octadecadienoic acid (KODA). Importantly, both 12-OPDA and KODA provided enhanced resistance to C. graminicola in a dose-dependent manner when transfused with xylem sap collected from untreated maize (See Figure). Moreover, 12-OPDA restored ISR activity in plants colonized by Tv-Δsm1 and in lox10 mutants, supporting the idea that 12-OPDA is a critical signal for long-distance ISR signaling in maize.
Since 12-OPDA is a precursor of the defense hormone jasmonic acid (JA), the authors investigated the relevance of bioactive jasmonic acid-isoleucine (JA-Ile) during ISR in maize. Phenotypic analysis of the JA-deficient opr7 opr8 double mutant demonstrated that JA itself was dispensable for ISR. Moreover, increasing doses of JA-Ile led to enhanced susceptibility when transfused with maize xylem sap. Collectively, the data suggested that the accumulation of 12-OPDA rather than JA-Ile is required for the establishment of T. virens-induced ISR in maize. In support of this idea, transcriptome analysis of maize plants undergoing ISR revealed that 12-OPDA and KODA biosynthesis genes were induced upon T. virens colonization, whereas JA-related genes were ultimately repressed. Together, the data illustrate a directed effort to accumulate the mobile-oxylipin signals 12-OPDA and KODA during ISR. Further efforts to understand the contribution of these oxylipins to ISR in maize and other angiosperms will provide fundamental insight into long-distance immune signaling activated by beneficial interactions with rhizosphere microbes.
Philip Carella
Sainsbury Laboratory
University of Cambridge,
Cambridge, United Kingdom
ORCID: 0000-0002-5467-7290
REFERENCES
Constantino NN, Matsouri F, Damarwinasis R, Borrego EJ, Moran-Diez ME, Kenerley CM, Gao X and Kolomiets MV (2013). Root-expressed maize lipoxygenase 3 negatively regulates induced systemic resistance to Colletotrichum graminicola in shoots. Front. Plant Sci. 4: 510. doi: 10.3389/fpls.2013.00510.
Djonović S, Vargas WA, Kolomiets MV, Horndeski M, Wiest A and Kenerley CM (2007). A proteinaceous elicitor Sm1 from the beneficial fungus Trichoderma virens is required for induced systemic resistance in maize. Plant Physiol. 145: 875-889
Pieterse CMJ, Zamioudis C, Berendsen RL, Weller DM, Van Weese SCM and Bakker PAHM (2014) Induced systemic resistance by beneficial microbes. Ann. Rev. Phytopath. 52: 347-375.
Wang KD, Borrego EJ, Kenerley CM and Molomiets MV (2019). Oxylipins other than jasmonic acid are xylem-resident signals regulating systemic resistance induced by Trichoderma virens in maize. Plant Cell Published November 2019 DOI: https://doi.org/10.1105/tpc.19.00487