Viruses on the move in the extracellular space
Lynn GL Richardson
Department of Plant Biology, Michigan State University, East Lansing, MI 48824
Positive strand (+) RNA viruses act as their own messengers, encoding an RNA-dependent RNA polymerase that can replicate their sense RNA genome. This allows (+)RNA viruses to replicate outside the nucleus, within replication complexes formed from host organelle membranes often called “replication factories” (Laliberte and Zheng, 2014; Romero-Brey and Bartenschlager, 2014; Shulla and Randall, 2016). Replication complexes of the plant (+) RNA virus Turnip mosaic virus (TuMV) are derived from higher order vesiculation of ER membranes and formation of multivesicular bodies (MVBs), which contain numerous virus-containing intraluminal vesicles (Grangeon et al., 2013; Wan et al., 2015). TuMV vesicles and MVBs can move from cell to cell through plasmodesmata, and aggregated replication factories have been found in the xylem of infected plants, with both routes contributing to the spread of the virus within the plant (Grangeon et al., 2013; Wan et al., 2015; Wan and Laliberté, 2015).
Through investigating host cell factors involved in the maturation of TuMV replication complexes, a MVB-localized SNARE (Soluble NSF Attachment protein Receptor) protein, Vti11 (Vps10(ten) interacting protein 11), was shown to play a critical role in TuMV replication and systemic infection in Arabidopsis thaliana (Cabanillas et al., 2018). This same SNARE was also found within the proteome of extracellular vesicles (EVs) isolated from Arabidopsis. In eukaryotic cells EVs are derived from several sources, including apoptotic bodies formed via blebbing from the cell during programmed cell death, microsomes formed from plasma membrane vesiculation, and exosomes, which are derived from MVB intraluminal vesicles that are released upon fusion of the MVB boundary membrane with the plasma membrane (Rutter and Innes, 2018). Given its localization to MVBs, Vti11 likely also functions in exosome biogenesis by mediating fusion of MVBs with the plasma membrane. The dual functions of Vti11 in TuMV infection and MVB-plasma membrane fusion potentially hint toward an extracellular location for viral vesicles through fusion of TuMV MVBs with the plasma membrane. This would represent a novel pathway for intercellular movement of plant viruses.
In this issue of Plant Physiology, Mohaved et al. (2019) set out to investigate the possible existence of TuMV replication complexes in the extracellular space. Nicotiana benthamiana leaves were agroinfiltrated with an infectious cDNA version of TuMV that expresses the membrane-bound viral protein 6K2 fused to GFP. Confocal microscopy revealed 6K2-positive aggregates that localized to the extracellular space, delineated by the FM4-64-stained plasma membrane (Figure 1A). A closer look using transmission electron microscopy (TEM) showed that MVB-plasma membrane fusion events occurred at significantly higher levels in infected leaves, and the number of vesicles present in the extracellular space was also significantly higher in infected leaves. Immunogold labeling of double-stranded RNA (dsRNA), which should be present within viral replication complexes, demonstrated that EVs, as well as MVBs that could be observed fusing with the plasma membrane, contained dsRNA (Figure 1B). These observations are consistent with labelled EVs representing TuMV replication complexes that are derived from viral MVB fusion with the plasma membrane.
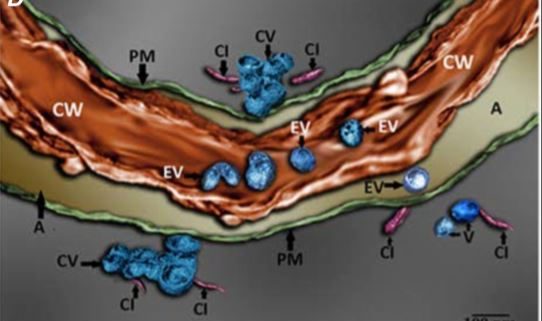
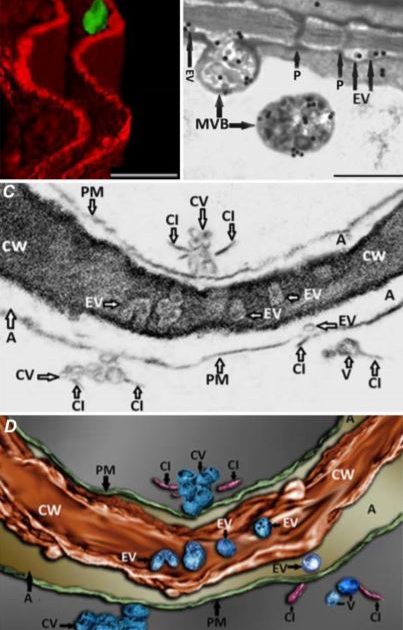 It was also apparent from TEM images that viral EVs may exist within the cell wall, although this is difficult to clearly discern from two-dimensional TEM images due to the high electron density of the cell wall. The authors used focused ion beam–extreme high-resolution scanning electron microscopy (FIB–EHRSEM) to obtain more precise structural details of the cell wall and any associated vesicles in infected cells. This technique uses a focused ion beam to section the sample while images are collected using scanning electron microscopy (SEM) (Figure 1C). Images of 250 slices of 7nm each were used to construct a 3D rendered image of a TuMV-infected cell, specifically focusing on the extracellular region. The reconstruction clearly shows several spherical EVs within the cell wall (Figure 1D), suggesting that TuMV EVs are able to penetrate and likely cross the cell wall. This is the first evidence to suggest the existence of replication complexes within the extracellular space and cell wall, and suggests a novel mechanism for viral cell-to-cell spread. Given the similarities in replication mechanisms with other (+) RNA viruses that infect a wide diversity of hosts, from economically important crop plants to humans and other animals (Ahlquist et al., 2003; Laliberte and Zheng, 2014), this may represent an important common pathway for other (+) RNA virus replication and spread.
It was also apparent from TEM images that viral EVs may exist within the cell wall, although this is difficult to clearly discern from two-dimensional TEM images due to the high electron density of the cell wall. The authors used focused ion beam–extreme high-resolution scanning electron microscopy (FIB–EHRSEM) to obtain more precise structural details of the cell wall and any associated vesicles in infected cells. This technique uses a focused ion beam to section the sample while images are collected using scanning electron microscopy (SEM) (Figure 1C). Images of 250 slices of 7nm each were used to construct a 3D rendered image of a TuMV-infected cell, specifically focusing on the extracellular region. The reconstruction clearly shows several spherical EVs within the cell wall (Figure 1D), suggesting that TuMV EVs are able to penetrate and likely cross the cell wall. This is the first evidence to suggest the existence of replication complexes within the extracellular space and cell wall, and suggests a novel mechanism for viral cell-to-cell spread. Given the similarities in replication mechanisms with other (+) RNA viruses that infect a wide diversity of hosts, from economically important crop plants to humans and other animals (Ahlquist et al., 2003; Laliberte and Zheng, 2014), this may represent an important common pathway for other (+) RNA virus replication and spread.
Relative to humans and other higher vertebrates, less is known about exosomes in plants, although recent advances in isolating plant EVs have led to a greater understanding of their physiological importance (Rutter and Innes, 2017, 2018; Rybak and Robatzek, 2019). In plants, EVs are known to contain antimicrobial compounds and defense-related proteins, and participate in localized immune responses to invading fungal and bacterial pathogens (Rutter and Innes, 2018). In the current study (Mohaved et al., 2019) the proteome of infected N. benthamiana and Arabidopsis EVs was investigated using improved EV isolation methods (Rutter and Innes, 2017). Among those host proteins found exclusively in EVs of infected plants, several were identified that are known to be involved in TuMV replication, as well as enzymes involved in lipid and secondary metabolism biosynthesis pathways. Several additional host proteins whose involvement in TuMV infection is not yet characterized were also observed, and should be promising candidates for further investigation. These exciting data pave the way for future studies aimed at understanding how exosomes contribute to systemic viral infection in plants and other organisms. TuMV EV biogenesis may also serve as a model system for further discovery of the molecular players involved in this vesicle transport pathway in plants.
Figure Legends
Figure 1. Imaging of TuMV extracellular vesicles in infected N. benthamiana leaves. A) 3D reconstruction from confocal micrographs of leaves infected with TuMV encoding GFP-tagged 6K2. The plasma membrane was stained with the endocytic dye FM4-64 (red). Scale bar = 2 µM. B) Infected leaves were immunolabelled with nanogold particles that detect double-stranded RNA, a marker for replicating virus. Scale bar = 500 nm. C) A single image obtained using focused ion beam–extreme high-resolution scanning electron microscopy (FIB–EHRSEM), focusing on the extracellular space between two juxtaposed TuMV-infected cells. Scale same as in D. D) 3D rendered image using FIB-EHRSEM, showing vesicles in the extracellular space and cell wall. Scale bar = 100 nm. MVB; multivesicular body; Chl, chloroplast; EV, extracellular vesicle; P, plasmodesmata; PM, plasma membrane; CV, cluster of vesicles; CI, cylindrical inclusion body; CW, cell wall; A, apoplast. Images adapted from Figures 1, 3 and 4 in Mohaved et al., 2019.
References
Ahlquist P, Noueiry AO, Lee WM, Kushner DB, Dye BT (2003) Host factors in positive-strand RNA virus genome replication. J Virol 77: 8181-8186
Cabanillas DG, Jiang J, Movahed N, Germain H, Yamaji Y, Zheng H, Laliberte JF (2018) Turnip Mosaic Virus Uses the SNARE Protein VTI11 in an Unconventional Route for Replication Vesicle Trafficking. Plant Cell 30: 2594-2615
Edgar JR (2016) Q&A: What are exosomes, exactly? BMC Biol 14: 46
Grangeon R, Jiang J, Wan J, Agbeci M, Zheng H, Laliberte JF (2013) 6K2-induced vesicles can move cell to cell during turnip mosaic virus infection. Front Microbiol 4: 351
Laliberte JF, Zheng H (2014) Viral Manipulation of Plant Host Membranes. Annu Rev Virol 1: 237-259
Meckes DG, Jr. (2015) Exosomal communication goes viral. J Virol 89: 5200-5203
Mohaved N, Cabanillas DG, Wan J, Vali H, Laliberté JF, Zheng H. 2019. Turnip mosaic virus components are released into the extracellular space by vesicles in infected leaves. Plant Physiol. DOI 10.1104/pp.19.00381
Romero-Brey I, Bartenschlager R (2014) Membranous replication factories induced by plus-strand RNA viruses. Viruses 6: 2826-2857
Rutter BD, Innes RW (2017) Extracellular Vesicles Isolated from the Leaf Apoplast Carry Stress-Response Proteins. Plant Physiol 173: 728-741
Rutter BD, Innes RW (2018) Extracellular vesicles as key mediators of plant-microbe interactions. Curr Opin Plant Biol 44: 16-22
Rybak K, Robatzek S (2019) Functions of Extracellular Vesicles in Immunity and Virulence. Plant Physiol 179: 1236-1247
Shulla A, Randall G (2016) (+) RNA virus replication compartments: a safe home for (most) viral replication. Curr Opin Microbiol 32: 82-88
Wan J, Basu K, Mui J, Vali H, Zheng H, Laliberte JF (2015) Ultrastructural Characterization of Turnip Mosaic Virus-Induced Cellular Rearrangements Reveals Membrane-Bound Viral Particles Accumulating in Vacuoles. J Virol 89: 12441-12456
Wan J, Cabanillas DG, Zheng H, Laliberte JF (2015) Turnip mosaic virus moves systemically through both phloem and xylem as membrane-associated complexes. Plant Physiol 167: 1374-1388
Wan J, Laliberté JF (2015) Membrane-associated virus replication complexes locate to plant conducting tubes. Plant Signal Behav 10: e1042639