Tracking the Courier: In Planta Imaging of NADH/NAD+ Ratios with a Genetically Encoded Biosensor
The movement of electrons between molecules and organelles via redox reactions is a cornerstone of cellular metabolism. Electron carriers such as nicotinamide adenine dinucleotide (NAD) and its phosphorylated form NADP mediate reduction or oxidation reactions via their own conversion between reduced [NAD(P)H] and oxidized [NAD(P)+] forms. Altered NAD levels lead to impaired plant development and stress responses due to its manifold roles in signaling (Gakière et al., 2018). The NAD pool size and the ratios of its reduced and oxidized forms change in response to environmental cues. Unfortunately, monitoring these changes has been challenging due to the lack of in vivo analysis techniques in plants. In this issue, Steinbeck et al. (2020) demonstrate that Peredox-mCherry, a pH-insensitive fluorescent biosensor of NADH/NAD+ ratio (Hung et al., 2011), can be applied in plants to detect NADH/NAD+ ratios in different tissues and to monitor their change in response to environmental triggers.
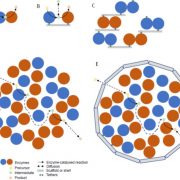
 Peredox-mCherry is a ratiometric sensor that contains two NADH or NAD+ binding domains fused with the fluorescent reporters tSapphire and mCherry; binding of NADH, but not NAD+, to the sensor protein leads to a conformational change and increased tSapphire fluorescence without affecting the internal reference, mCherry fluorescence (Hung et al., 2011). Thus, the tSapphire/mCherry fluorescence ratio reports the NADH/NAD+ ratio. Steinbeck et al. (2020) generated stable Arabidopsis thaliana (Arabidopsis) lines expressing Peredox-mCherry in the cytosol and showed that sensor expression did not have adverse effects on plant growth. The authors confirmed that the sensor was largely stable across a pH range of 6 to 9 and that it specifically detected NAD and not NADP redox ratio under physiological conditions.
Peredox-mCherry is a ratiometric sensor that contains two NADH or NAD+ binding domains fused with the fluorescent reporters tSapphire and mCherry; binding of NADH, but not NAD+, to the sensor protein leads to a conformational change and increased tSapphire fluorescence without affecting the internal reference, mCherry fluorescence (Hung et al., 2011). Thus, the tSapphire/mCherry fluorescence ratio reports the NADH/NAD+ ratio. Steinbeck et al. (2020) generated stable Arabidopsis thaliana (Arabidopsis) lines expressing Peredox-mCherry in the cytosol and showed that sensor expression did not have adverse effects on plant growth. The authors confirmed that the sensor was largely stable across a pH range of 6 to 9 and that it specifically detected NAD and not NADP redox ratio under physiological conditions.
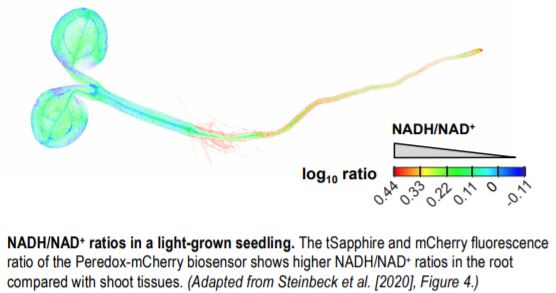
These sensor lines were used to characterize NADH/NAD+ ratios in different tissues of light- and dark-grown seedlings. The authors found more reduced NAD pools in roots compared to shoot tissues (see figure) of seedlings grown in the light, whereas NADH/NAD+ ratios were lower in dark-grown seedlings, with the most pronounced oxidation of the NAD pool in the roots.
The authors then studied the effects of light on cytosolic NADH/NAD+ ratios in leaf disks of fully grown plants. In response to light, the cytosolic NAD pool was reduced, but not in the presence of a photosynthetic electron transport inhibitor. Thus, chloroplast electron transport led to NAD reduction in the cytosol in the light, possibly due to export of reductant from the chloroplast via carbon metabolites. External sucrose supplementation that should increase NADH production via glycolysis and the tricarboxylic acid cycle, and inhibition of mitochondrial electron transport (a major sink for NADH), both led to an increased NADH/NAD+ ratio in the cytosol. Therefore, Peredox-mCherry can detect changes in NAD redox ratios in the cytosol in response to altered metabolism, making it a valuable tool to study reductant flows between organelles.
The authors noticed that Peredox-mCherry signals were close to saturation under various experimental treatments in leaf disks, potentially hindering its use in tissues with more reduced NAD pools. To tackle this issue, the authors engineered a mutant version of the sensor that had ~25 times lower NADH binding affinity but maintained pH stability and specificity of NAD sensing over NADP. This modified sensor (Peredox-mCherry DS) measured a lower steady-state NADH/NAD+ ratio than Peredox-mCherry under the same conditions and extended the sensor response range to the inhibition of mitochondrial electron transport, supporting its potential use for detecting larger changes in NADH/NAD+ ratios.
With this genetically encoded, pH-insensitive NAD redox state sensor in plants, Steinbeck et al. (2020) have made it possible to live track a key metabolic courier in subcellular redox messaging, which should lead to a better understanding of its roles in metabolism and signaling.
Hanna Hõrak
Institute of Technology
University of Tartu, Estonia
ORCID: 0000-0002-6392-859X
REFERENCES
Gakière, B., Fernie, A.R., and Pétriacq, P. (2018). More to NAD+ than meets the eye: A regulator of metabolic pools and gene expression in Arabidopsis. Free Radical Biology and Medicine 122: 86–95.
Hung, Y.P., Albeck, J.G., Tantama, M., and Yellen, G. (2011). Imaging cytosolic NADH-NAD+ redox state with a genetically encoded fluorescent biosensor. Cell Metabolism 14: 545–554.