How Plants Clear Toxic Proteins
Gil et al. explore ZTL-mediated protein quality control https://doi.org/10.1105/tpc.17.00612
By Kyung-Eun Gil
Background: As sessile organisms, plants have evolved various mechanisms to adapt to environmental changes. Under stressful conditions such as high temperatures, proteins are misfolded and undergo denaturation. The denatured proteins form toxic aggregates in the cell. These toxic protein aggregates must be repaired or broken down. Heat shock proteins (HSPs) are well-known molecular chaperones that function in protein renaturation and clearance together with ubiquitin ligases. However, which ubiquitin ligases are important for degrading protein aggregates in plants is currently unclear.
Question: We would like to know how these protein aggregates are cleared through the ubiquitin-proteasomal pathway. The main question is this: Which ubiquitin ligase plays a role in degrading protein aggregates at high temperatures?
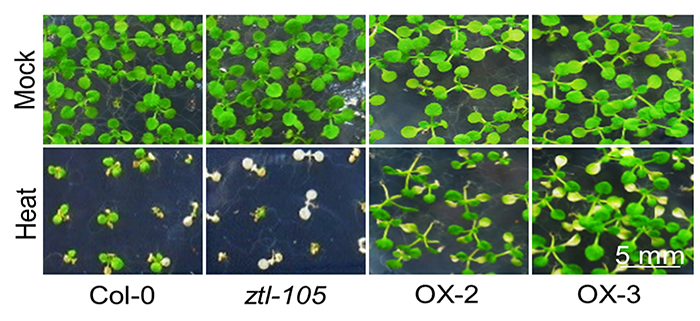
Findings: We found that ZEITLUPE (ZTL) works together with the molecular chaperone HSP90 in clearing protein aggregates at high temperatures. We found that the Arabidopsis mutant, ztl-105, is sensitive in heat stress, while overexpression of ZTL enhances the plant’s tolerance to heat stress. Also, we found that ztl-105 has reduced amounts of ubiquitinated proteins after heat shock, indicating that the lack of ZTL causes insufficient ubiquitination of denatured protein, which in turn increases the levels of protein aggregates in ztl-105. We also observed reduced ubiquitination and increased levels of protein aggregates in HSP90 knock-down plants, supporting our idea that ZTL and HSP90 function together in degrading protein aggregates at high temperatures. In addition, we made the important discovery that ZTL helps maintain the circadian clock at high temperatures.
Next steps: Future research will focus on the formation and/or degradation of insoluble proteins after abiotic stress. The two main questions are as follows: Which proteins primarily form protein aggregates? Which ubiquitin ligases and HSPs are important for protein quality control under various abiotic stress conditions?
Kyung-Eun Gil, Woe-Yeon Kim, Hyo-Jun Lee, Mohammad Faisal, Quaiser Saquib, Abdulrahman Alatar, and Chung-Mo Park (2017) ZEITLUPE contributes to a thermoresponsive protein quality control system in Arabidopsis. 29: 2882-2894 https://doi.org/10.1105/tpc.17.00612