Too Active in ER Fusion? Here Comes LUNAPARK to the Rescue
Sun et al. found that Arabidopsis LUNAPARK, a protein evolutionarily conserved in eukaryotes, is an E3 ligase that promotes the degradation of ROOT HAIR DEFECTIVE3, an ER fusogen. Plant Cell https://doi.org/10.1105/tpc. 18.00937
By Jiaqi Sun, Hugo Zheng, Department of Biology, McGill University, 1205 Dr Penfield Avenue, Montreal, H3A 1B1, Quebec, Canada
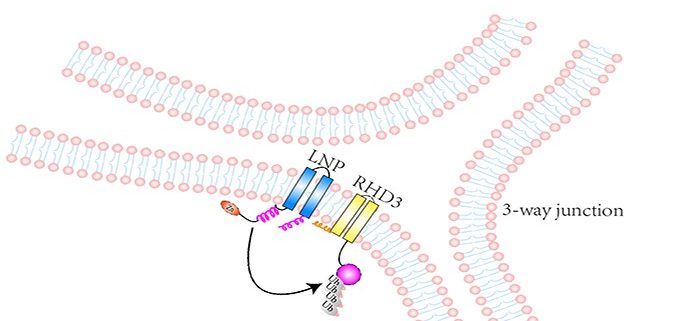
Background: Plant cells have numerous membrane-bound compartments in which many biological reactions critical for proper cell development and function occur. The endoplasmic reticulum (ER) is a compartment that is organized into a netlike labyrinth of interconnected tubules and flattened sheets in which proteins and lipids are synthesized and processed. The formation and maintenance of this labyrinth-like structure requires fusion of different ER tubules. These fusion events are mediated by ROOT HAIR DEFECTIVE3 (RHD3). Because uncontrolled action of RHD3 would lead to excessive fusion, the action of RHD3 must be tightly regulated. Otherwise, abnormal ER will be formed in cells, leading to improper cell development and/or cell responses to stresses.
Questions: Is the action of RHD3 regulated by other factors, and if so, how is the activity of RHD3 regulated?
Findings: We first found that RHD3 physically interacts with two proteins called LUNAPARK1 (LNP1) and LUNAPARK2 (LNP2) in Arabidopsis. After the fusion of different ER tubules is completed, RHD3 recruits either LNP1 or LNP2 to a so-called 3-way junction site of the ER where different ER tubules fuse. Subsequently, RHD3 disappears from the site. In this way, a newly formed 3-way junction site becomes stable. How do LNPs make RHD3 disappear? We found that both LNP1 and LNP2 can act as E3 ligases to ubiquitinate RHD3, which is then degraded. In Arabidopsis lnp1 lnp2 double mutant cells, RHD3 can not be degraded efficiently, leading to unstable newly formed 3-way junctions and sheet-like ER.
Next steps: LNPs form an evolutionarily conserved family of proteins, but there is no typical E3 domain in LNPs. Our next questions to answer are: 1) How do LNPs function as E3 ligases?; 2) Do LNPs act on other ER-shaping proteins?; and 3) Are LNPs involved in ER autophagy so to maintain ER homeostasis?
Jiaqi Sun, Nooshin Movahed, and Huanquan Zheng. (2020). LUNAPARK Is an E3 Ligase that Mediates Degradation of ROOT HAIR DEFECTIVE3 to Maintain a Tubular ER Network in Arabidopsis. Plant Cell. https://doi.org/10.1105/tpc.18.00937