TOC-TIC supercomplex structure
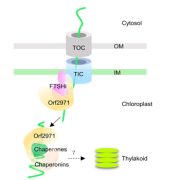
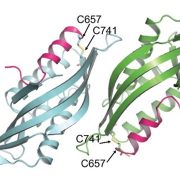
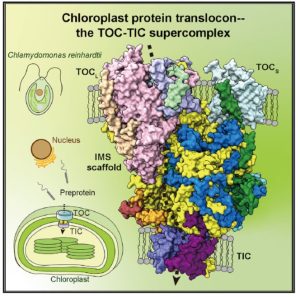 In an evolutionary plot twist, most of the proteins needed inside the chloroplast are encoded in the nucleus and translated in the cytosol as preproteins. The preproteins are imported into the chloroplast through two membranes (outer and inner). Genetic and biochemical approaches have revealed many of the components of the multi-protein complexes that allow this import, known as TOC (translocon outer membrane chloroplast) and TIC (translocon inner membrane chloroplast; the phylogenetically distant structures with similar function in mitochondria are known as TOM and TIM). Now, the awesome power of cryo-EM has revealed the structures of these complexes from Chlamydomonas in outstanding detail, providing new insights into how protein import is conducted. The work reveals that the membrane-spanning channel of TOC is made up of β-barrel pores whereas that of TIC mainly consists of transmembrane helices. Furthermore, TOC and TIC are held together into a supercomplex by an intermembrane space (IMS) scaffold that has a hydrophilic cleft that likely guides the translocation of the preprotein. The authors note that some of the proteins that make up the TOC-TIC supercomplex are restricted to certain lineages; for example, the IMS scaffold components are absent in some Poaceae species including wheat, rice and maize, raising questions of what this structure looks like in those species. (Summary by Mary Williams @PlantTeaching) Cell 10.1016/j.cell.2022.10.030
In an evolutionary plot twist, most of the proteins needed inside the chloroplast are encoded in the nucleus and translated in the cytosol as preproteins. The preproteins are imported into the chloroplast through two membranes (outer and inner). Genetic and biochemical approaches have revealed many of the components of the multi-protein complexes that allow this import, known as TOC (translocon outer membrane chloroplast) and TIC (translocon inner membrane chloroplast; the phylogenetically distant structures with similar function in mitochondria are known as TOM and TIM). Now, the awesome power of cryo-EM has revealed the structures of these complexes from Chlamydomonas in outstanding detail, providing new insights into how protein import is conducted. The work reveals that the membrane-spanning channel of TOC is made up of β-barrel pores whereas that of TIC mainly consists of transmembrane helices. Furthermore, TOC and TIC are held together into a supercomplex by an intermembrane space (IMS) scaffold that has a hydrophilic cleft that likely guides the translocation of the preprotein. The authors note that some of the proteins that make up the TOC-TIC supercomplex are restricted to certain lineages; for example, the IMS scaffold components are absent in some Poaceae species including wheat, rice and maize, raising questions of what this structure looks like in those species. (Summary by Mary Williams @PlantTeaching) Cell 10.1016/j.cell.2022.10.030