Tic-Tac-Toe: How TIC and TOC Coordinate Getting Proteins Across the Line
Most proteins within a cell are encoded in the nucleus and then translated in the cytosol, but how do they end up where they need to be? With the exception of the few proteins expressed within the chloroplast, the process of shipping nucleus-encoded proteins into the chloroplast is dependent on N-terminal transit peptides. The chloroplast protein import machinery recognizes these peptide tags and assists in translocating the proteins across the outer and inner chloroplast envelope. Some of these transported proteins can be further targeted to other suborganellar compartments such as the inner envelope membrane, thylakoid membrane, and thylakoid lumen.
Many steps of chloroplast protein import have been elucidated based on in vitro binding studies and the energetics of protein import, but several questions on the interactions of preproteins and how all the components contribute to the translocation remain unanswered. Richardson et al. (2018) take steps to fill this gap by investigating the coordinated activity of translocons at the outer (TOC) and inner (TIC) chloroplast envelope membranes and mapping the molecular topology of the transit peptide.
The authors generated different versions of the Rubisco small subunit preprotein by removing all cysteine residues of the transit peptide and mature protein and targeting unique positions with a single cysteine residue fused with expression tags. Combining this with a photo-inducible, heterobifunctional crosslinker, site-specific crosslinking could be observed over the length of the transit peptide. This allowed them to determine what was happening at specific stages of import through the TOC/TIC channels and under specific conditions.
Based on their results, they hypothesize that in the early energy-independent stages of import, before the transit peptide is incorporated in the chloroplast import machinery, the TOC34 and TOC159 GTPase import receptors are GDP-loaded (Figure, panel A). The transit peptide of the protein interacts with the TOC GTPases, triggering changes in receptor dimerization and the promotion of GDP to GTP exchange (panel B). This results in the transit peptide being inserted into the TOC/TIC translocon, with the N-terminal region in contact with the TIC integral inner membrane protein, Tic20, and the mid-region of the transit peptide associated with the Toc75 TOC channel.
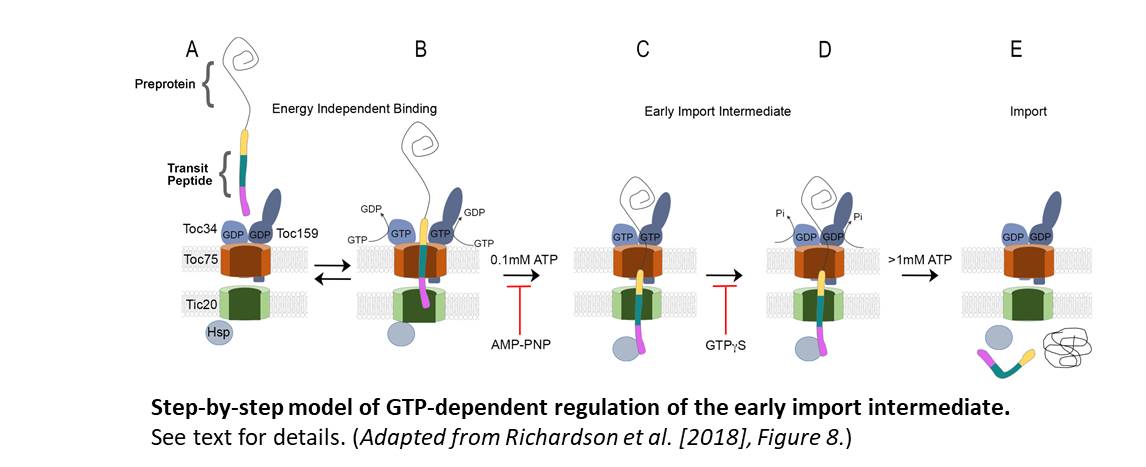
Low levels (~0.1 mM) of ATP promote the second stage of shuttling by stimulating the re-positioning of the transit peptide within the TOC and TIC channels. The N-terminus is exposed to the stroma, the mid-region of the transit peptide is inserted within the TIC channel, and the C-terminal region remains in contact with Toc75 (panel C).
GTP hydrolysis at the TOC GTPase import receptors then stabilizes the early import intermediate, the committed step in import (panel D). This is consistent with earlier observations that GTPγS inhibits the formation of the ATP-dependent early import intermediate and that GTP binding and receptor dimerization enhance import efficiency, as shown by Aronsson et al. (2010). Finally, the complete import of the preprotein into the stroma requires high levels of ATP (>1 mM), facilitated by the ATPase activity of stromal chaperone motors (Hsp) (panel E).
A significant finding by Richardson et al. is that the transit peptide crossed the TOC channel and engaged the TIC channel (Tic20) in the absence of NTP hydrolysis. Consequently, NTP hydrolysis was not required for the initial recognition and the early stages of import. Rather, the authors hypothesize that GTP and ATP hydrolysis enables the transition from reversible recognition of the transit peptide to protein import into the chloroplast. In this scenario, GTP hydrolysis acts as a checkpoint on whether or not to proceed, and ATP provides the energy for protein import.
Richardson et al. provide a model for translocation at the molecular level and insight into the NTP-dependent mechanisms of protein translocation in chloroplasts. The balancing act of GTP and ATP hydrolysis and transit peptide interactions with the translocation machinery during import provide an outline for future studies aimed at identifying functional motifs within the transit peptide.
REFERENCES
Aronsson, H., Combe, J., Patel, R., Agne, B., Martin, M., Kessler, F., and Jarvis, P. (2010). Nucleotide binding and dimerization at the chloroplast pre-protein import receptor, at Toc33, are not essential in vivo but do increase import efficiency. Plant J. 63: 297-311.
Richardson, L.G., Small, E. L., Inoue, H., and Schnell, D.J. (2018). Molecular topology of the transit peptide during chloroplast protein import. Plant Cell https://doi.org/10.1105/tpc.18.00172.