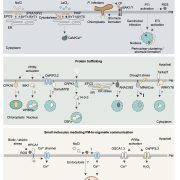
A Lipid Droplet-Associated Degradation System in Plants
Once viewed merely as inert packets of metabolic energy that are mobilized during postgerminative growth, lipid droplets (LDs) have emerged as dynamic organelles with important roles in processes ranging from stress responses to hormone signaling (Pyc et al., 2017). LDs consist of a core of neutral lipids surrounded by a phospholipid monolayer harboring specific structural proteins, predominantly oleosins in seeds and pollen. These LD-associated proteins stabilize LDs and prevent LD fusion, and are important regulators of LD dynamics; their ubiquitination, extraction, and proteasomal degradation precede LD breakdown (D’Andrea, 2016; Deruyffelaere et al., 2015). However, little is known about the mechanism regulating the extraction and turnover of LD-associated proteins in plants.
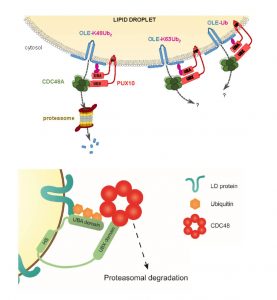 In companion papers published in this issue of The Plant Cell, two independent teams present important insights into this mechanism (Deruyffelaere et al., 2018; Kretzschmar et al., 2018). Having previously identified PLANT UBX DOMAIN-CONTAINING PROTEIN 10 (PUX10) and the AAA-type ATPase CELL DIVISION CYCLE 48 (CDC48A; a key component of ubiquitin-mediated pathways (Baek et al., 2013)) as candidates functioning in the degradation of ubiquitinated oleosins (Deruyffelaere et al., 2015), Deruyffelaere et al. (2018) investigated the role of these two proteins in oleosin turnover during seedling germination. Kretzschmar et al. (2018) analyzed the LD-associated proteome of Nicotiana tabacum pollen tubes to gain insight into LD biosynthesis and degradation. In addition to identifying PUX10, Kretzschmar et al. (2018) spotted several other LD-associated proteins (Müller et al., 2017), and confirmed their LD association by expressing them in pollen tubes. They decided to explore the role of PUX10 in this study.
In companion papers published in this issue of The Plant Cell, two independent teams present important insights into this mechanism (Deruyffelaere et al., 2018; Kretzschmar et al., 2018). Having previously identified PLANT UBX DOMAIN-CONTAINING PROTEIN 10 (PUX10) and the AAA-type ATPase CELL DIVISION CYCLE 48 (CDC48A; a key component of ubiquitin-mediated pathways (Baek et al., 2013)) as candidates functioning in the degradation of ubiquitinated oleosins (Deruyffelaere et al., 2015), Deruyffelaere et al. (2018) investigated the role of these two proteins in oleosin turnover during seedling germination. Kretzschmar et al. (2018) analyzed the LD-associated proteome of Nicotiana tabacum pollen tubes to gain insight into LD biosynthesis and degradation. In addition to identifying PUX10, Kretzschmar et al. (2018) spotted several other LD-associated proteins (Müller et al., 2017), and confirmed their LD association by expressing them in pollen tubes. They decided to explore the role of PUX10 in this study.
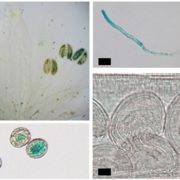
Experiments using a variety of overlapping and distinct molecular and cell biology techniques and experimental systems (including Arabidopsis seedlings, N. benthamiana leaves, and N. tabacum pollen tubes) revealed that PUX10 is anchored in the LD monolayer via its hydrophobic domain and interacts with ubiquitin via its UBA domain and with CDC48A via its UBX domain. Mutation of PUX10 had striking effects on LD size and morphology during germination (Deruyffelaere et al., 2018; Kretzschmar et al., 2018), impairing the degradation of ubiquitinated oleosins and blocking their extraction from LDs (Deruyffelaere et al., 2018). Intriguingly, PUX10 associated with only a subset of LDs, suggesting that distinct populations of LDs exist in plants.
Based on their findings, the researchers arrived at similar conclusions (see figure) regarding a mechanism underlying the degradation of LD-associated proteins, which Deruyffelaere et al. (2018) named the LD-associated degradation (LDAD) system. In essence, the teams propose that PUX10 connects CDC48A with ubiquitinated LD-associated proteins in the LD monolayer, which promotes extraction of the LD-associated proteins from the LD and facilitates their proteasomal degradation.
These results represent substantial progress in the field of plant LD biology and set the stage for future studies of LD dynamics. Work is already underway to determine if other proteins are part of the LDAD system, and what their roles in LD-associated protein turnover may be.
References
Baek, G.H., Cheng, H., Choe, V., Bao, X., Shao, J., Luo, S., and Rao, H. (2013). Cdc48: a swiss army knife of cell biology. J Amino Acids 2013, 183421.
Pyc, M., Cai, Y., Greer, M.S., Yurchenko, O., Chapman, K.D., Dyer, J.M., Mullen, R.T. (2017). Turning Over a New Leaf in Lipid Droplet Biology. Trends Plant Sci 22: 596-609.
D’Andrea, S. (2016). Lipid droplet mobilization: The different ways to loosen the purse strings. Biochimie 120 :17-27.
Deruyffelaere, C., Bouchez, I., Morin, H., Guillot, A., Miquel, M., Froissard, M., Chardot, T., and D’Andrea, S. (2015). Ubiquitin-Mediated Proteasomal Degradation of Oleosins is Involved in Oil Body Mobilization During Post-Germinative Seedling Growth in Arabidopsis. Plant Cell Physiol. 56, 1374-1387.
Deruyffelaere, C., Purkrtova, Z., Bouchez, I., Collet, B., Cacas, J.-L., Chardot, T., Gallois, J.L., D’Andrea, A. (2018). PUX10 is a CDC48A Adaptor Protein that Regulates the Extraction of Ubiquitinated Oleosins from Seed Lipid Droplets in Arabidopsis. Plant Cell tpc.00275.2018; DOI: 10.1105/tpc.18.00275.
Kretzschmar, F.K., Mengel, L.F., Müller, A., Schmitt, K., Blersch, K.F., Valerius, O., Braus, G., and Ischebeck, T. (2018). PUX10 is a Lipid Droplet-localized Scaffold Protein that Interacts with CELL DIVISION CYCLE 48 and is Involved in the Degradation of Lipid Droplet Proteins. Plant Cell tpc.00276.2018; DOI: 10.1105/tpc.18.00276.
Müller, A. O., Blersch, K. F., Gippert, A. L., and Ischebeck, T. (2017) Tobacco pollen tubes – a fast and easy tool to study lipid droplet association of plant proteins. Plant J 89, 1055-1064.