The Meaning of an End: N-terminal Acetyltransferase NAA50 Controls Plant Growth and Stress Responses
Author: Sjon Hartman
ORCID: 0000-0002-6709-6436
Plant Ecophysiology, Institute of Environmental Biology, Utrecht University, Padualaan 8, 3584 CH, Utrecht, The Netherlands
At least 80% of eukaryotic proteins are estimated to undergo N-terminal acetylation (NTA), making it likely that your favorite protein is also regulated by NTA (Linster and Wirtz, 2018). This transfer of an acetyl group to the N-terminus can modulate a protein’s interaction partners, folding, localization, aggregation and degradation (Gibbs, 2015). NTA is catalyzed by several distinct N-terminal acetyltransferase (Nat) complexes (NatA, NatB, NatC and NatE), which each modify different N-terminal amino acids and consist of catalytic and auxiliary subunits. For instance, the catalytic subunits of NatA (NAA10) and NatE (NAA50) in humans target a distinct set of substrates but share the same ribosome binding auxiliary subunit, facilitating co-translational acetylation of target proteins. However, such characterization of individual Nat subunits in plants is lacking and currently hinders our understanding of how NTA regulates biological processes.
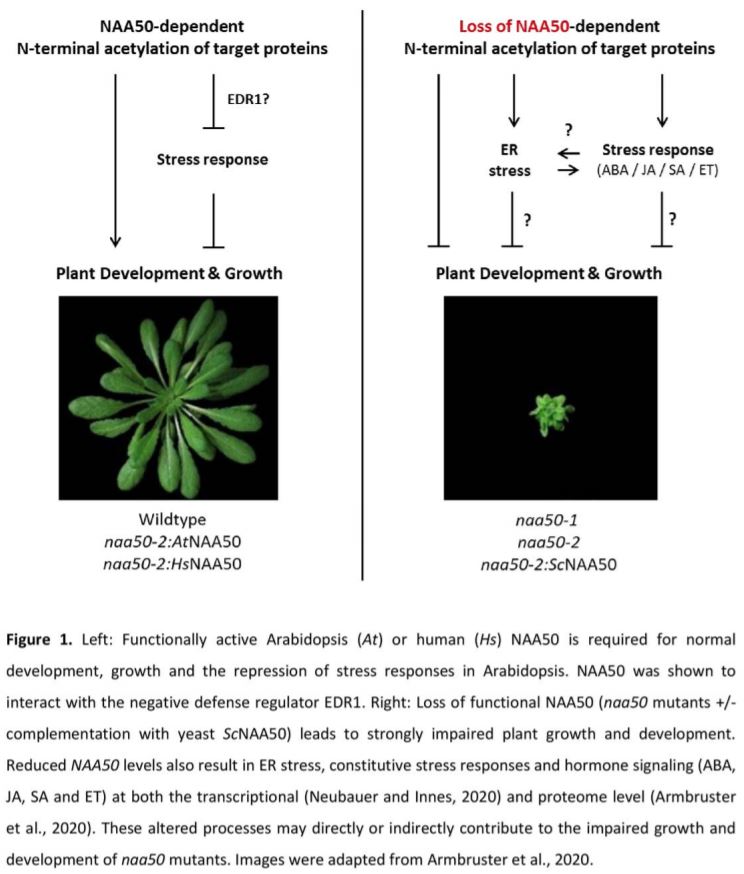 In this issue of Plant Physiology, two back-to-back publications characterize the Arabidopsis (Arabidopsis thaliana) catalytic NatE subunit NAA50 (Armbruster et al., 2020; Neubauer and Innes, 2020). Together the papers show that NAA50 localizes to the nucleus, cytosol and the endoplasmic reticulum (ER) and acetylates a broad range of N-terminal substrates in vitro. Moreover, they show that naa50 mutants are strongly limited in plant growth, fertility and development (Fig. 1). These findings are consistent with an additional recent report showing that NAA50 is essential for plant growth (Feng et al., 2020). To examine if the distorted growth of naa50 mutants is caused by a loss of functional NAA50, the authors complemented the mutants with several modified versions of NAA50. Expression of Arabidopsis NAA50 fully restored the growth and developmental deficiencies of the mutant. Furthermore, Armbruster et al. (2020) show that NAA50 function is conserved between humans and plants, as even the human homolog of NAA50 could complement naa50. They could also map this activity to a conserved NAA50 region that is only present in higher eukaryotes, as yeast NAA50 could not rescue the impaired growth phenotype. In yeast, NAA50 is essential to position the NatA complex at the exit tunnel of the ribosome (Knorr et al., 2019). However, in the current study no abnormalities in the acetylation of known NatA substrates were identified in naa50 (Armbruster et al., 2020). Collectively, these findings strongly suggest that Arabidopsis NAA50 is an enzymatically active NatE but does not contribute to NatA activity.
In this issue of Plant Physiology, two back-to-back publications characterize the Arabidopsis (Arabidopsis thaliana) catalytic NatE subunit NAA50 (Armbruster et al., 2020; Neubauer and Innes, 2020). Together the papers show that NAA50 localizes to the nucleus, cytosol and the endoplasmic reticulum (ER) and acetylates a broad range of N-terminal substrates in vitro. Moreover, they show that naa50 mutants are strongly limited in plant growth, fertility and development (Fig. 1). These findings are consistent with an additional recent report showing that NAA50 is essential for plant growth (Feng et al., 2020). To examine if the distorted growth of naa50 mutants is caused by a loss of functional NAA50, the authors complemented the mutants with several modified versions of NAA50. Expression of Arabidopsis NAA50 fully restored the growth and developmental deficiencies of the mutant. Furthermore, Armbruster et al. (2020) show that NAA50 function is conserved between humans and plants, as even the human homolog of NAA50 could complement naa50. They could also map this activity to a conserved NAA50 region that is only present in higher eukaryotes, as yeast NAA50 could not rescue the impaired growth phenotype. In yeast, NAA50 is essential to position the NatA complex at the exit tunnel of the ribosome (Knorr et al., 2019). However, in the current study no abnormalities in the acetylation of known NatA substrates were identified in naa50 (Armbruster et al., 2020). Collectively, these findings strongly suggest that Arabidopsis NAA50 is an enzymatically active NatE but does not contribute to NatA activity.
To uncover how loss of NAA50 represses growth, Neubauer and Innes (2020) quantified the transcriptome of a hormone-inducible NAA50 amiRNA line and Armbruster et al. (2020) examined the proteome in an naa50 mutant. Both approaches revealed that loss of NAA50 leads to constitutive ER stress and (a)biotic stress responses. The enhanced defense response also corresponded with increased signaling of the stress-associated hormones salicylic acid (SA), jasmonic acid (JA), abscisic acid (ABA) and ethylene (JA) (Fig. 1). In addition, Neubauer and Innes (2020) show that NAA50 interacts with the negative stress regulator ENHANCED DISEASE RESISTANCE 1 (EDR1), providing a potential mechanism for how NAA50 dampens defense responses. The authors suggest that the observed ER stress could be the result of accumulating unfolded and aggregated proteins in the naa50 mutant. Interestingly, previous research has shown that crosstalk between ER stress and the defense response can regulate the tradeoff between plant growth and stress responses (Meng et al., 2017). Indeed, SA can divert energy from fueling plant growth to the amelioration of ER stress, while ER stress in turn switches on defense responses (Meng et al., 2017; Srivastava et al., 2018). Together, these observations suggest that the reduced growth of naa50 mutants depends on a shifting growth–defense balance that may depend on crosstalk between ER stress and defense responses (Fig. 1). To unravel if the naa50-impaired growth is indeed the result of constitutively induced defense hormone signaling, future experiments could attempt to rescue the growth phenotype by crossing naa50 with the corresponding hormone biosynthesis and signaling mutants.
The current work convincingly adds another piece to the complex NTA puzzle and allows us to examine what proteins directly undergo co-translational NTA by NAA50 and identify how NAA50 dynamics modulate specific stress responses. Other recent studies also highlight the importance of post-translational NTA at the plasma membrane and in plastids (Bienvenut et al., 2020; Linster et al., 2020). Collectively, these reports demonstrate that NTA and Nat complexes deserve our full attention when we aim to understand the regulation of plant growth, development and stress responses (Feng et al., 2020; Huber et al., 2020).
LITERATURE CITED
Bienvenut WV, Brünje A, Boyer JB, Mühlenbeck JS, Bernal G, Lassowskat I, Dian, C Linster E, Dinh TV, Koskela MM, Jung V, Seidel J, Schyrba LK, Ivanauskaite A, Eirich J, Hell R, Schwarzer D, Mulo P, Wirtz M, Meinnel T, Giglione C, Finkemeier I (2020). Dual lysine and N-terminal acetyltransferases reveal the complexity underpinning protein acetylation. Molecular Systems Biology, in press, DOI: 10.15252/msb.20209464.
Gibbs DJ (2015) Emerging Functions for N-Terminal Protein Acetylation in Plants. Trends Plant Sci 20: 599–601
Feng J, Hu J, Li Y, Li R, Yu H, Ma L (2020) The N-Terminal Acetyltransferase Naa50 Regulates Arabidopsis Growth and Osmotic Stress Response. Plant Cell Physiol. DOI: 10.1093/PCP/PCAA081
Huber M, Bienvenut W V., Linster E, Stephan I, Armbruster L, Sticht C, Layer D, Lapouge K, Meinnel T, Sinning I, et al (2020) NatB-mediated N-terminal acetylation affects growth and biotic stress responses. Plant Physiol 182: 792–806
Knorr AG, Schmidt C, Tesina P, Berninghausen O, Becker T, Beatrix B, Beckmann R (2019) Ribosome–NatA architecture reveals that rRNA expansion segments coordinate N-terminal acetylation. Nat Struct Mol Biol 26: 35–39
Linster E, Wirtz M (2018) N-terminal acetylation: An essential protein modification emerges as an important regulator of stress responses. J Exp Bot 69: 4555–4568
Linster E, Layer D, Bienvenut WV, Dinh-Van T, Weyer FA, Leemhuis W, Brünje A, Hoffrichter M, Miklankova P, Kopp J, Lapouge K, Sindlinger J, Schwarzer D, Meinnel T, Finkemeier I, Giglione C, Hell R, Sinning I, Wirtz M. (2020). The Arabidopsis Na-acetyltransferase NAA60 locates to the plasma membrane and is vital for the high salt stress response. New Phytologist, in press, DOI: 10.1111/nph.16747.
Meng Z, Ruberti C, Gong Z, Brandizzi F (2017) CPR5 modulates salicylic acid and the unfolded protein response to manage tradeoffs between plant growth and stress responses. Plant J 89: 486–501
Srivastava R, Li Z, Russo G, Tang J, Bi R, Muppirala U, Chudalayandi S, Severin A, He M, Vaitkevicius SI, et al (2018) Response to Persistent ER Stress in Plants: A Multiphasic Process That Transitions Cells from Prosurvival Activities to Cell Death. Plant Cell 30: 1220–1242