The calcium-permeable channel OSCA1.3 regulates plant stomatal immunity (Nature)
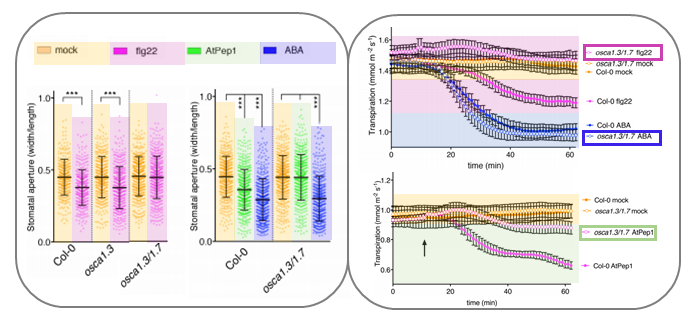
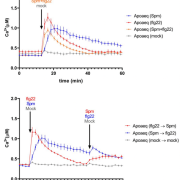
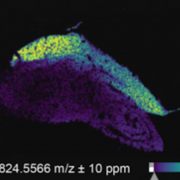
In plants, the perception of environmental threats induces a peak of calcium ions (Ca2+) in the cytosol that triggers signal transduction pathways leading to stomatal closure as defense response. In Arabidopsis, the mechanosensitive Ca2+ channel OSCA1 regulates water transpiration in response to abiotic stress, yet, a similar Ca2+channel involved in plant response to pathogens has not been identified. In this article, Thor and coworkers discovered that OSCA1.3, an uncharacterized isoform localized to the plasma membrane, is essential for plant immunity. Upon treatment with elicitors (i.e., pathogen- and damage- associated molecular patterns, PAMPs and DAMPs), the plasma membrane-associated cytosolic kinase BIK1 activates OSCA1.3 by directly phosphorylating its first cytoplasmic loop. Furthermore, electrophysiological experiments revealed that OSCA1.3 facilitates Ca2+ transport, as its expression in multiple heterologous systems correlates with an increase in cytosolic Ca2+. The authors also observed that the loss of function mutations of OSCA1.3 and the related gene OSCA1.7 associates with reduced Ca2+ spiking in guard cells, and consequently impairment of stomatal closure after treatment with elicitors (flagellin22 and AtPep1) but not with the abiotic stress hormone ABA. To summarize, Ca2+ channels play essential roles in the regulation of stomata functioning to guarantee gas exchange for optimal photosynthesis as well as prompt response to abiotic and biotic stresses. Specifically, OSCA1 and OSCA1.3/OSCA1.7 control stomata closing to reduce water loss and to restrict pathogen entry, respectively. (Summary and image adaptation by Michela Osnato @michela_osnato) Nature 10.1038/s41586-020-2702-1