Structural basis of salicylic acid perception by Arabidopsis NPR proteins (Nature) ($)
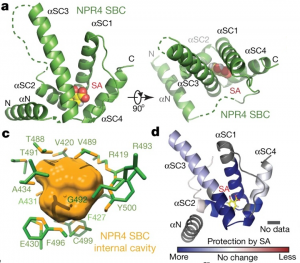 Salicylic acid (SA) is a critical hormone in plant-pathogen responses. The main receptors of this hormonal signal are a small family of NPR proteins (NONEXPRESSOR OF PATHOGENESIS-RELATED GENES). Although NPR1 is a positive regulator of defense signaling, NPR3 and NPR4 serve as negative regulators; they also interact with NPR1 in a SA-mediated manner. Previous efforts to identify the crystal structure of the full-length NPR-SA complex have been unsuccessful. Here, Wang et al. crystalized just the SA-binding core of NPR4 (amino acids 373 to 516) bound to SA. Notably, the structure showed that SA is completely buried within the binding site with no obvious entry point. This finding suggests that there may be a major conformational remodeling of NPR4 upon SA binding. This was confirmed by hydrogen deuterium-exchange mass spectrometry analysis Interestingly, although NPR1 and NPR4 share very similar hormone-binding residues, NPR1 shows much less SA-binding activity when compared to NPR4. Finally, the authors showed that expression of an NPR4 SA hypersensitive variant enhances SA-mediated basal immunity but without affecting its interaction with NPR1 in high levels of SA. With this understanding of SA signaling mechanisms, it could be possible to engineer plants with boosted plant immunity. (Summary by Camila Ribeiro @camicribeiro86) Nature 10.1038/s41586-020-2596-y
Salicylic acid (SA) is a critical hormone in plant-pathogen responses. The main receptors of this hormonal signal are a small family of NPR proteins (NONEXPRESSOR OF PATHOGENESIS-RELATED GENES). Although NPR1 is a positive regulator of defense signaling, NPR3 and NPR4 serve as negative regulators; they also interact with NPR1 in a SA-mediated manner. Previous efforts to identify the crystal structure of the full-length NPR-SA complex have been unsuccessful. Here, Wang et al. crystalized just the SA-binding core of NPR4 (amino acids 373 to 516) bound to SA. Notably, the structure showed that SA is completely buried within the binding site with no obvious entry point. This finding suggests that there may be a major conformational remodeling of NPR4 upon SA binding. This was confirmed by hydrogen deuterium-exchange mass spectrometry analysis Interestingly, although NPR1 and NPR4 share very similar hormone-binding residues, NPR1 shows much less SA-binding activity when compared to NPR4. Finally, the authors showed that expression of an NPR4 SA hypersensitive variant enhances SA-mediated basal immunity but without affecting its interaction with NPR1 in high levels of SA. With this understanding of SA signaling mechanisms, it could be possible to engineer plants with boosted plant immunity. (Summary by Camila Ribeiro @camicribeiro86) Nature 10.1038/s41586-020-2596-y