A protein engineering approach for elucidating receptor protein signaling function (Plant Cell)
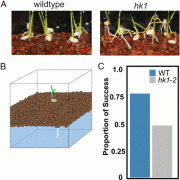
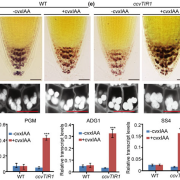
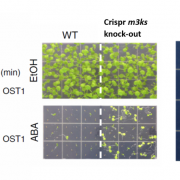
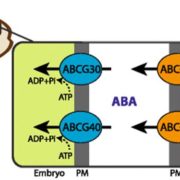
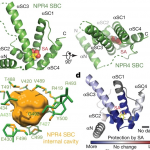
 Plant cells express an array of membrane-bound receptor proteins that recognize and respond to extracellular cues. However, their varied structure/specificity and methods of activation pose challenges in assessing their function. Focusing on Leucine-Rich Repeat Receptor Kinases (LRR-RKs), Hohmann et al. used a protein engineering approach to generate chimeras for functional elucidation. Many LRR-RKs require association with a co-receptor SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE (SERK) to trigger intracellular signaling upon recognition of a ligand. BAK1-INTERACTING RECEPTOR-LIKE KINASES (BIRs) compete with LRR-RKs to bind with the extracellular domain of SERK, independent of any ligands. This competition in effect shuts off the signaling. The authors tried to induce constitutive, ligand-independent expression of several signaling pathways by combining the extracellular and transmembrane domains of a BIR receptor protein (which bind to SERK) and the intracellular kinase domains of different SERK-dependent LRR-RKs, which initiate signaling. These chimeras induced constitutive activation in brassinosteroid signaling, floral abscission, stomatal development, and Casparian strip formation, revealing that this LRR-RK/SERK/BIR interaction is present in a variety of tissues. This “…strategy allows for the identification of gain-of-function phenotypes of orphan LRR-RKs whose ligands are unknown, and enables the elucidation of their receptor activation mechanism.” (Summary by Benjamin Jin) Plant Cell 10.1105/tpc.20.00138
Plant cells express an array of membrane-bound receptor proteins that recognize and respond to extracellular cues. However, their varied structure/specificity and methods of activation pose challenges in assessing their function. Focusing on Leucine-Rich Repeat Receptor Kinases (LRR-RKs), Hohmann et al. used a protein engineering approach to generate chimeras for functional elucidation. Many LRR-RKs require association with a co-receptor SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE (SERK) to trigger intracellular signaling upon recognition of a ligand. BAK1-INTERACTING RECEPTOR-LIKE KINASES (BIRs) compete with LRR-RKs to bind with the extracellular domain of SERK, independent of any ligands. This competition in effect shuts off the signaling. The authors tried to induce constitutive, ligand-independent expression of several signaling pathways by combining the extracellular and transmembrane domains of a BIR receptor protein (which bind to SERK) and the intracellular kinase domains of different SERK-dependent LRR-RKs, which initiate signaling. These chimeras induced constitutive activation in brassinosteroid signaling, floral abscission, stomatal development, and Casparian strip formation, revealing that this LRR-RK/SERK/BIR interaction is present in a variety of tissues. This “…strategy allows for the identification of gain-of-function phenotypes of orphan LRR-RKs whose ligands are unknown, and enables the elucidation of their receptor activation mechanism.” (Summary by Benjamin Jin) Plant Cell 10.1105/tpc.20.00138