Gatekeepers of Storage Lipids in Seeds: The Switch-Off Mechanism
Deruyffelaere et al. investigate lipid mobilization during seed germination. Plant Cell https://doi.org/10.1105/tpc.18.00275
By Sabine D’ANDREA and Corine ENARD
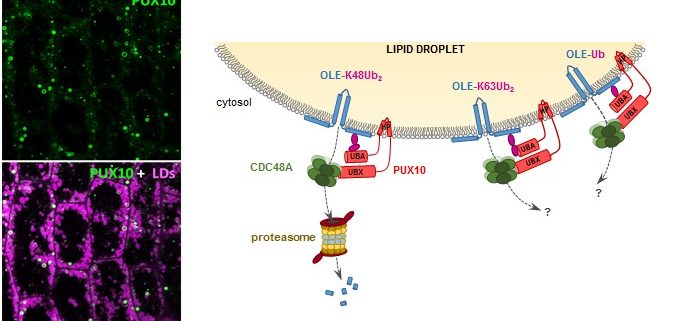
Background: In cells, storage lipids are compartmentalized in specialized organelles called lipid droplets (LDs). The structure of these compartments is conserved across kingdoms and unique among organelles, consisting of a phospholipid monolayer containing proteins and surrounding the storage lipid core. LDs accumulate in many seeds, including those of oilseed crops (soybean, rapeseed, and sunflower). In dry seeds, LDs remain stable for years. After seed germination, LDs are degraded to provide carbon and energy for seedling growth. We know relatively little about how quiescent LDs are turned into dynamic ones during seed germination. Oleosins, the major proteins associated with LDs in seeds, are crucial for LD stability: They control LD size and prevent LD fusions during seed desiccation. In germinating seeds, oleosins are degraded before the onset of lipid mobilization.
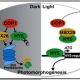
Question: Prior to degradation, oleosins are marked for destruction by ubiquitination, a modification in which they are tagged with a small protein called ubiquitin. Wondering how ubiquitinated oleosins are removed from the LD surface to allow LD degradation during seed germination, we searched for factors involved in extracting oleosins from the LD coat.
Findings: We found that a protein called PUX10 is required for proper extraction of ubiquitinated oleosins from the LD surface in young Arabidopsis seedlings. PUX10 localizes to LDs and binds to ubiquitinated proteins. PUX10 also interacts with CDC48A, the ATPase that selectively extracts misfolded proteins from the ER in the degradation process known as ERAD (for ER-associated degradation). We propose that PUX10 is an adaptor protein that recruits CDC48A to ubiquitinated oleosins, thus promoting the extraction of oleosins from LDs by the segregase activity of CDC48A. PUX10 and CDC48A are core components of a novel LD-associated machinery we named the LD-associated degradation (LDAD) system. Surprisingly, PUX10 and CDC48A localize to a specific subpopulation of LDs in germinated seeds, suggesting a functional differentiation of LDs in plants.
Next steps: We will focus on identifying and characterizing additional proteins of the LDAD machinery. These could be involved in events such as controlling ubiquitin chain modification or recruiting specific degradation systems that recognize the ubiquitin mark borne by oleosins.
Carine Deruyffelaere, Zita Purkrtova, Isabelle Bouchez, Boris Collet, Jean-Luc Cacas, Thierry Chardot, Jean-Luc Gallois, Sabine D’Andrea. (2018). PUX10 Is a CDC48A Adaptor Protein That Regulates the Extraction of Ubiquitinated Oleosins from Seed Lipid Droplets in Arabidopsis. Plant Cell 30: 2116-2136; DOI: https://doi.org/10.1105/tpc.18.00275
Keywords : seed germination, lipid, oleosin, ubiquitination