Stem diameter fluctuations provide a new window into plant water status and function
Author: Robert Skelton
Affiliation: South African Environmental Observation Network, Private Bag X7, Claremont, 7735, South Africa
Knowledge of the spatio-temporal dynamics of water storage in plant stems is fundamental to understanding plant physiological function under drought stress (Tyree and Yang 1990; Holbrook 1995; Skelton 2019). Usually such processes—including reversible living-cell dehydration-rehydration and capillary discharge of water from dead xylem vessels—are as opaque to observers as wood itself. However, in this volume of Plant Physiology an exciting study by Lamacque et al. (2020) examines whether changes in branch diameter can provide a useful proxy of plant water status, thus providing an insightful window into the dynamics of water discharge in stems during water deficit.
 Relating changes in stem diameter to plant water status is a complex challenge because variation in diameter is caused by irreversible radial growth in addition to water movement. To separate these processes, Lamacque et al. (2020) conducted two controlled drought-re-watering experiments with varying drought stress intensity, assuming that growth would be limited during drought phases. Next, the authors developed novel quantitative metrics of stem diameter change, including the percent loss of recovery potential (defined as the difference between maximum pre-drought and post-rehydration diameters) and percent loss of diameter (defined as the difference between maximum pre-drought and minimum drought diameters), and related these to plant water potential and electrolyte leakage. Overall, their results show that the relationship between percent loss of stem diameter and xylem water potential is non-linear and that complete loss of recovery potential (i.e. plant death) was more closely associated with high levels of cell damage than to xylem hydraulic failure.
Relating changes in stem diameter to plant water status is a complex challenge because variation in diameter is caused by irreversible radial growth in addition to water movement. To separate these processes, Lamacque et al. (2020) conducted two controlled drought-re-watering experiments with varying drought stress intensity, assuming that growth would be limited during drought phases. Next, the authors developed novel quantitative metrics of stem diameter change, including the percent loss of recovery potential (defined as the difference between maximum pre-drought and post-rehydration diameters) and percent loss of diameter (defined as the difference between maximum pre-drought and minimum drought diameters), and related these to plant water potential and electrolyte leakage. Overall, their results show that the relationship between percent loss of stem diameter and xylem water potential is non-linear and that complete loss of recovery potential (i.e. plant death) was more closely associated with high levels of cell damage than to xylem hydraulic failure.
Changes in stem diameter are not linearly related to changes in xylem water potential in part because water is stored in several tissues within stems and these sources release their water at different times during dehydration. Lamacque et al. (2020) showed that lavender stem diameters shrink rapidly at relatively high water potentials (i.e. less negative than ~−2 or −3 MPa), but shrink less rapidly as the plants dry out (see Figure 5 in Lamacque et al. (2020)). Although Lamacque et al. (2020) do not identify the specific tissue supplying the moisture during each phase, this process has been examined in previous studies. After observing a lack of discharge of stored stem water prior to vessel cavitation in American chestnut (Castanea dentata) seedlings, Knipfer et al. (2019) concluded that early changes in stem diameter during the onset of water deficit are likely driven primarily by bark shrinkage. As the whole plant dries out, water stored in these non-xylem tissues (i.e. the bark) can be released into the xylem, slowing the rate of change of xylem water potential (i.e. decreasing the rate of decline of xylem water potential).
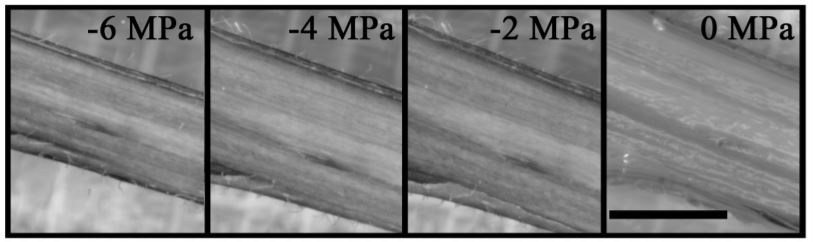
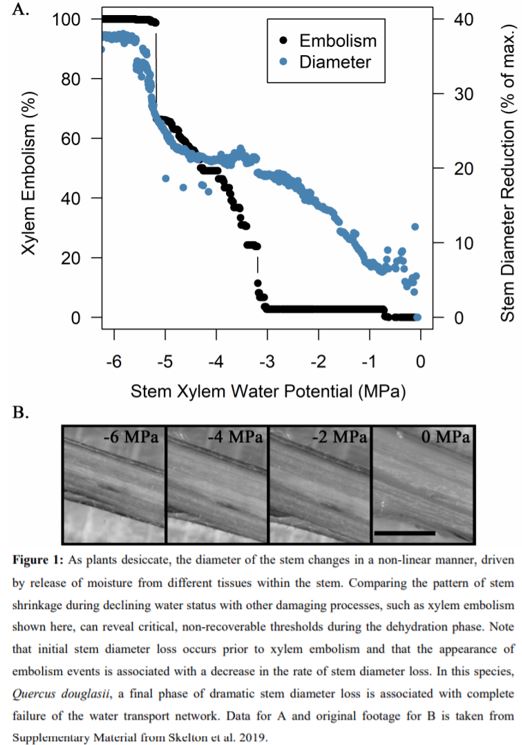
Elastic release of stored stem water from living tissues embedded within the xylem matrix to neighboring xylem conduits (e.g. vessels) would also slow the rate of change of xylem water potential. Such elastic release of water from living cells within the xylem matrix has been suggested to occur later (i.e. at lower water potentials), often simultaneously with the appearance of xylem embolism (Knipfer et al. 2020). However, optical data from oak species (see data from Quercus douglasii in Figure 1) indicate that living tissues within the xylem matrix shrink simultaneously to the bark, and before embolism, suggesting that this process might be species specific.
A third explanation for the non-linear relationship between water potential and stem diameter is that capillary discharge of water from xylem vessels during the process of xylem cavitation (see Figure 1A) or from dead tissue in the xylem matrix (e.g. dead fibers) can reduce the rate of stem diameter shrinkage if the water is subsequently taken up by living cells. Early embolism events are associated with a decrease in the rate of stem diameter loss in long vessel oaks (see Figure 1A). This stored water is not used to avoid xylem embolism nor to provide water to the transpiration stream, as very little capillary water is released prior to stomatal closure (Knipfer et al. 2019). Instead, the release of water stored in the dead tissue in the xylem matrix contributes to the hydration of cambial meristem tissue, possibly serving to promote cambial viability during desiccation and promote whole-plant survival (Knipfer et al. 2019; Lamacque et al. 2020).
A second insight from Lamacque et al. (2020) is that lavender plant water status (inferred from changes in stem diameter) is non-linearly related to ability to recover from dehydration (see Figure 3 in Lamacque et al. 2020). Such results are clearly important for revealing critical points during the dehydration phase where even small changes in stem diameter can be associated with substantial, irreversible loss of stem function following rehydration. Overall, it emerges that there are several phases to plant water status dynamics occurring in a predictable sequence. The first phase is a dynamic, recoverable change in stem diameter driven by release of moisture from bark. This is followed by loss of rehydration recovery potential driven by an inability to refill cavitated vessels (i.e. an inability to replace water lost through capillary discharge). Later loss of recovery potential is associated with elastic discharge from meristem tissues. Finally, complete loss of recovery potential occurs when the water storage of the elastic compartment of the branch is exhausted.
Lamacque et al. (2020) show that in lavender plants this final phase occurs beyond 88% loss of stem hydraulic conductance and is coupled with high electrolyte leakage, leading them to conclude that plant death was not linked to xylem hydraulic failure but rather to high levels of cell damage. Results from other species indicate that these two processes are intricately linked. For example, results from North American oaks suggest that complete stem shrinkage (i.e. total capillary and elastic discharge) occurs simultaneously with 100% embolism (e.g. Quercus douglasii shown in Figure 1A and B; see also supplementary material in Skelton et al. 2019). Further, Knipfer et al. (2019) found that water released by cavitation may make an important contribution to the survival of trees during drought events by protecting cambial viability, suggesting that the relationships between discharge from the conducting elements, recovery potential, and capacity to resprout from the cambial meristem are likely to be complex. Future studies might aim to further disentangle these relationships, thereby providing more clarity to the question of what kills plants during drought.
Acknowledgements
The author acknowledges the support of the FLAIR programme, Royal Society, and the African Academy of Sciences (Award number FLR\ R1\191609). The FLAIR Fellowship Programme is a partnership between the African Academy of Sciences and the Royal Society funded by the UK Government’s Global Challenges Research Fund.
References
Holbrook NM (1995) Stem water storage. In Gartner BL (ed) Plant stem: physiology and functional morphology. Academic Press, San Diego.
Knipfer T, Reyes C, Earles JM, Berry ZC, Johnson DM, Brodersen CR, McElrone AJ (2019) Spatiotemporal Coupling of Vessel Cavitation and Discharge of Stored Xylem Water in a Tree Sapling. Plant Physiol 179: 1658–1668
Skelton R (2019) Of storage and stems: Examining the role of stem water storage in plant water balance. Plant Physiol 179: 1433–1434
Skelton RP, Anderegg LDL, Papper P, Reich E, Dawson TE, Kling M, Thompson SE, Diaz J, Ackerly DD (2019) No local adaptation in leaf or stem xylem vulnerability to embolism, but consistent vulnerability segmentation in a North American oak. New Phytol nph.15886
Tyree MT, Yang S (1990) Water-storage capacity of Thuja, Tsuga and Acer stems measured by dehydration isotherms – The contribution of capillary water and cavitation. Planta 182: 420–426