Self-Incompatibility Can Cause Death in Vegetative Cells
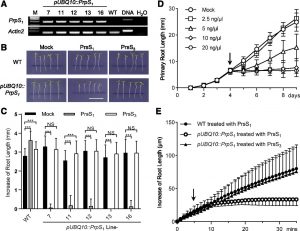 Self-incompatibility (SI) is a mechanism used by flowering plants to prevent self-fertilization. It is controlled by a multi-allelic S-locus that allows self/non-self-recognition between pistil and pollen. In several SI systems, when male and female S-determinants match, self pollen is recognized and rejected before fertilization can occur. A key characteristic of SI determinants is that they are extremely tightly regulated, both in a developmental and tissue-specific manner, being expressed solely in pistil and pollen cells during a narrow developmental window, as the tissues approach maturity. SI in poppy (Papaver rhoeas), for example, is controlled and specified by S determinants expressed specifically in the stigma (PrsS) and pollen (PrpS) respectively. PrpS encodes a small, novel, integral membrane protein with several predicted transmembrane domains; belongs to the cysteine-rich peptide family, whose members activate diverse signaling networks involved in plant growth, defense and reproduction. Interaction of cognate PrsS and PrpS triggers pollen tube growth inhibition and programmed cell death of ‘self’ pollen. Lin et al. (10.1104/pp.20.00292) now show that PrpS and PrsS, when expressed ectopically, act as a bipartite module to trigger a “self-recognition and self-destruct” response in Arabidopsis independently of reproductive context, that is, in vegetative cells. The addition of recombinant PrsS to seedling roots expressing the cognate PrpS resulted in hallmark features of the Papaver SI response, including S-specific growth inhibition and programmed cell death of root cells.. Moreover, inducible expression of PrsS in PrpS-expressing seedlings resulted in rapid death of the entire seedling. This demonstrates that, besides specifying SI, the bipartite PrpS–PrsS module can trigger growth arrest and cell death in vegetative cells.
Self-incompatibility (SI) is a mechanism used by flowering plants to prevent self-fertilization. It is controlled by a multi-allelic S-locus that allows self/non-self-recognition between pistil and pollen. In several SI systems, when male and female S-determinants match, self pollen is recognized and rejected before fertilization can occur. A key characteristic of SI determinants is that they are extremely tightly regulated, both in a developmental and tissue-specific manner, being expressed solely in pistil and pollen cells during a narrow developmental window, as the tissues approach maturity. SI in poppy (Papaver rhoeas), for example, is controlled and specified by S determinants expressed specifically in the stigma (PrsS) and pollen (PrpS) respectively. PrpS encodes a small, novel, integral membrane protein with several predicted transmembrane domains; belongs to the cysteine-rich peptide family, whose members activate diverse signaling networks involved in plant growth, defense and reproduction. Interaction of cognate PrsS and PrpS triggers pollen tube growth inhibition and programmed cell death of ‘self’ pollen. Lin et al. (10.1104/pp.20.00292) now show that PrpS and PrsS, when expressed ectopically, act as a bipartite module to trigger a “self-recognition and self-destruct” response in Arabidopsis independently of reproductive context, that is, in vegetative cells. The addition of recombinant PrsS to seedling roots expressing the cognate PrpS resulted in hallmark features of the Papaver SI response, including S-specific growth inhibition and programmed cell death of root cells.. Moreover, inducible expression of PrsS in PrpS-expressing seedlings resulted in rapid death of the entire seedling. This demonstrates that, besides specifying SI, the bipartite PrpS–PrsS module can trigger growth arrest and cell death in vegetative cells.



