Roles of the clock in controlling starch metabolism
Author: Masanori Izumi
Affiliation: Frontier Research Institute for Interdisciplinary Sciences, Tohoku University, Sendai 980–8578, Japan
Plants are photoautotrophs that grow via photosynthetic energy production. Under irradiation, chloroplasts in the plant’s green tissues convert light energy to chemical energy and assimilate atmospheric CO2 into sugars to support growth. Plants do not stop growing during the night, though—they accumulate a portion of the photosynthesis-derived sugars during the day and then consume them for night-time growth (Smith and Stitt, 2007). These sugars are stored as insoluble starch granules in many plant species, including Arabidopsis (Arabidopsis thaliana).
Studies using Arabidopsis starchless mutants, in which chloroplast starch-producing enzymes are deficient (Caspar et al., 1985; Lin et al., 1988), have demonstrated the importance of a continuous sugar supply throughout the entire day for plant growth. In starchless mutants, soluble sugars hyperaccumulate as the alternative to starch; however, they are quickly exhausted early in the night, thereby inducing sugar starvation later in the night (Gibon et al., 2004; Bläsing et al., 2005). This temporal sugar shortage interrupts nighttime growth and attenuates growth resumption the following dawn, resulting in a significant delay of growth (Smith and Stitt, 2007). Therefore, plants must control the pace of starch breakdown to avoid nocturnal sugar deficiency.
The circadian clock plays a role in controlling starch degradation. When Arabidopsis plants are suddenly exposed to an artificial, earlier dusk during growth under light/dark cycles, they avoid sugar shortage by slowing down the rate of starch breakdown during the night, indicating that plants anticipate the time until next dawn (Graf et al., 2010). The transcription factors CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) and LATE ELONGATED HYPOCOTYL (LHY) constitute the circadian clock oscillator (Mizoguchi et al., 2002), and cca1 lhy double mutants exhaust their starch supplies, and therefore experience sugar starvation, before dawn in a 12-h light/12-h dark period (Graf et al., 2010). Those findings reveal that plants control the pace of starch degradation via circadian clock-mediated anticipation of dawn (Graf and Smith, 2011).
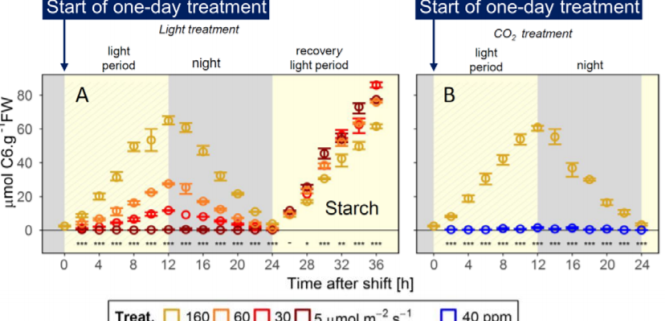
In nature, plants are exposed to fluctuating light conditions caused by the daily changes in weather, as well as shading from neighbors and wind-mediated changes in canopy structure. To avoid the nocturnal sugar starvation, plants must adjust the rate of starch breakdown to match the amount of starch acquired by diel photosynthesis, which changes day to day. In the current issue of Plant Physiology, Moraes et al. (2019) assess the role of the circadian clock in controlling starch turnover in response to the amount of diurnally assimilated starch. The authors established an experimental system that mimics the decrease in photosynthetic product due to environmental factors: Arabidopsis plants grown under 12 h-light at 160 µmol m-2 s-1 are subjected to one-day treatments with reduced light intensity (60, 30 or 5 µmol m-2 s-1) or reduced CO2 concentration, without an alteration in the light period.
Those treatments induced an elegant gradient of daytime increases and nighttime decreases in leaf starch contents (Figure 1). With the reduction of light intensity, the rate of starch accumulation during the day was reduced, and starch breakdown during the night also slowed down. The nocturnal decrease in starch content occurred in a linear manner under conditions of moderately low light (60 and 30 µmol m-2 s-1), thereby avoiding complete exhaustion of starch. The low CO2 concentration (40 ppm vs. 400 ppm in the control conditions) mimicked severe sugar starvation and helped the authors distinguish the effect of altered light signaling from the effects of reduced light intensity.
The authors integrated large datasets of diel changes in metabolites related to photosynthetic products and transcripts related to the circadian clock during the one-day treatments described above (Figure 1). Some circadian clock components showed consistent changes in transcript accumulation in response to the moderate reduction of starch levels due to lower light intensity; for instance, EARLY FLOWERING 4 (ELF4), a component of evening complex in Arabidopsis circadian clocks, was downregulated. Additionally, during the one-day treatment, reduced starch levels were accompanied by higher expression of REVEILLEs (RVEs) during the light period and of PHYTOCHROME INTERACTING FACTORs (PIFs) during the dark period. These changes might be an output of the slightly modulated ELF4 expression since the evening complex serves as a suppressor of PIF transcripts (Huang and Nusinow, 2016). However, the changes in clock-related transcripts in response to altered starch levels were small compared to the magnitude of the diel oscillation of clock components. In addition, the changes in PIF and RVE expression due to reduced starch availability were larger than those of the circadian clock components, leading the authors to clock-independent upregulation for PIFs and RVEs. Overall, the authors concluded that a circadian clock-independent system controls starch turnover in response to day-to-day changes in the amounts of photosynthetic products. This conclusion is consistent with the previously proposed model of arithmetic division (the AD model), in which the rate of starch breakdown is set by integrating the starch amount and the period to dawn as parallel inputs (Scialdone and Howard, 2015).
 However, the data of Moraes et al. are also consistent with an alternative model for daily changes in starch dynamics. The RMS model posits that the circadian oscillator is regulated by feedback signaling reflecting the status of sucrose, which is a product of starch breakdown (Seki et al., 2017). Notably, this model also predicts changes in starch dynamics in response to low light without a requirement for a change in phase of the circadian oscillator (Seki et al., 2017), indicating that the RMS model can account for the small changes in the circadian clock and large changes in starch turnover observed during the one-day treatments of the current study.
However, the data of Moraes et al. are also consistent with an alternative model for daily changes in starch dynamics. The RMS model posits that the circadian oscillator is regulated by feedback signaling reflecting the status of sucrose, which is a product of starch breakdown (Seki et al., 2017). Notably, this model also predicts changes in starch dynamics in response to low light without a requirement for a change in phase of the circadian oscillator (Seki et al., 2017), indicating that the RMS model can account for the small changes in the circadian clock and large changes in starch turnover observed during the one-day treatments of the current study.
It is also not known how plants sense the amount of total photosynthetic products to determine the rate of nighttime starch breakdown. Can plants directly estimate the amount of insoluble starch? Are there any signal molecules? Intriguingly, trehalose-6 phosphate acts as a signal reflecting energy status (Figueroa and Lunn, 2016), and an increase in trehalose-6 phosphate suppresses the rate of starch degradation (Martins et al., 2013; dos Anjos et al., 2018). Such signaling molecules might play an important role in day-to-day regulation of starch turnover. How plants sense daily outputs of photosynthesis, and adjust sugar metabolism, is an important question that awaits further characterization.
REPUBLISHED FROM https://doi.org/10.1104/pp.19.00166
LITERATURE CITED
Bläsing OE, Gibon Y, Gunther M, Hohne M, Morcuende R, Osuna D, Thimm O, Usadel B, Scheible WR, Stitt M (2005) Sugars and circadian regulation make major contributions to the global regulation of diurnal gene expression in Arabidopsis. Plant Cell 17: 3257-3281
Caspar T, Huber SC, Somerville C (1985) Alterations in growth, photosynthesis, and respiration in a starchless mutant of Arabidopsis thaliana (L.) deficient in chloroplast phosphoglucomutase activity. Plant Physiol 79: 11-17
dos Anjos L, Pandey PK, Moraes TA, Feil R, Lunn JE, Stitt M (2018) Feedback regulation by trehalose 6‐phosphate slows down starch mobilization below the rate that would exhaust starch reserves at dawn in Arabidopsis leaves. Plant Direct 2: e00078
Figueroa CM, Lunn JE (2016) A Tale of Two Sugars: Trehalose 6-Phosphate and Sucrose. Plant Physiol 172: 7-27 https://doi.org/10.1104/pp.18.01418
Gibon Y, Blasing OE, Palacios-Rojas N, Pankovic D, Hendriks JHM, Fisahn J, Hohne M, Gunther M, Stitt M (2004) Adjustment of diurnal starch turnover to short days: depletion of sugar during the night leads to a temporary inhibition of carbohydrate utilization, accumulation of sugars and post-translational activation of ADP-glucose pyrophosphorylase in the following light period. Plant J 39: 847-862
Graf A, Schlereth A, Stitt M, Smith AM (2010) Circadian control of carbohydrate availability for growth in Arabidopsis plants at night. Proc Natl Acad Sci U S A 107: 9458-9463
Graf A, Smith AM (2011) Starch and the clock: the dark side of plant productivity. Trends Plant Sci 16: 169-175
Huang H, Nusinow DA (2016) Into the Evening: Complex Interactions in the Arabidopsis Circadian Clock. Trends Genet 32: 674-686
Lin TP, Caspar T, Somerville C, Preiss J (1988) Isolation and characterization of a starchless mutant of Arabidopsis thaliana (L.) heynh lacking ADPglucose pyrophosphorylase activity. Plant Physiol 86: 1131-1135
Martins MCM, Hejazi M, Fettke J, Steup M, Feil R, Krause U, Arrivault S, Vosloh D, Figueroa CM, Ivakov A, Yadav UP, Piques M, Metzner D, Stitt M, Lunn JE (2013) Feedback Inhibition of Starch Degradation in Arabidopsis Leaves Mediated by Trehalose 6-Phosphate. Plant Physiol 163: 1142-1163
Mizoguchi T, Wheatley K, Hanzawa Y, Wright L, Mizoguchi M, Song HR, Carre IA, Coupland G (2002) LHY and CCA1 are partially redundant genes required to maintain circadian rhythms in Arabidopsis. Dev Cell 2: 629-641
Moraes TA, Mengin V, Annunziata MG, Encke B, Krohn N, Hoehne M, Stitt M (2019) Response of the circadian clock and diel starch turnover to one day of low light or low CO2. Plant Physiol
Scialdone A, Howard M (2015) How plants manage food reserves at night: quantitative models and open questions. Front Plant Sci 6: 204
Seki M, Ohara T, Hearn TJ, Frank A, da Silva VCH, Caldana C, Webb AAR, Satake A (2017) Adjustment of the Arabidopsis circadian oscillator by sugar signalling dictates the regulation of starch metabolism. Sci Rep 7: 8305
Smith AM, Stitt M (2007) Coordination of carbon supply and plant growth. Plant Cell Environ 30: 1126-1149