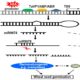
A New Component Regulating ABA Signaling
Wang et al. find that EAR1 is a general negative regulator of ABA signaling. https://doi.org/10.1105/tpc.17.00875
By Kai Wang, Jigang Li and Zhizhong Gong
Background: Abscisic acid (ABA) signaling in plants controls many biological processes, such as seed germination, seedling root growth, and stomatal movement. In the current understanding of ABA signaling, PYR/PYL/RCAR ABA receptor proteins, the clade A type 2C protein phosphatases (PP2Cs), and SNF1-related protein kinase 2s (SnRK2s) constitute the core components of the pathway. In the absence of ABA, PP2Cs negatively regulate the ABA signaling pathway by inhibiting the activity of SnRK2s; in the presence of ABA, the PYR/PYL/ACAR ABA receptors bind to and inhibit the phosphatase activity of PP2Cs, thus allowing the activation of SnRK2s and the phosphorylation of their target proteins. The ABA-binding affinities of some PYR/PYL/RCARs are enhanced when they interact with PP2Cs, therefore PP2Cs are also considered as ABA coreceptors.
Question: In addition to the PYR/PYL/ACAR ABA receptors, are there other components involved in regulating the function of PP2Cs, especially their phosphatase activity?
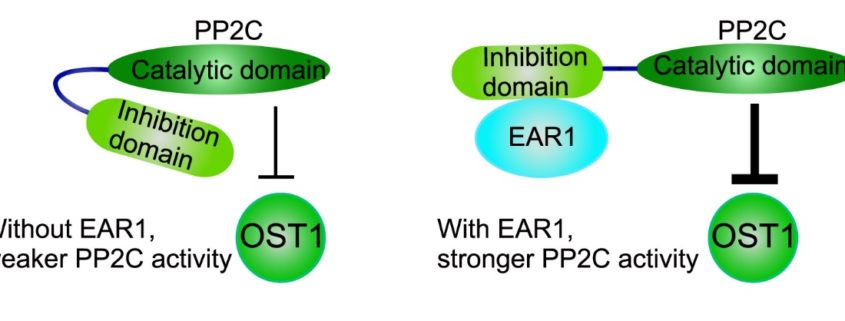
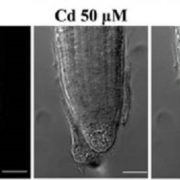
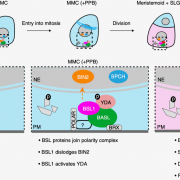
Findings: We identified a new component named EAR1 (ENHANCER OF ABA CO-RECEPTOR 1) that works in concert with clade A PP2C proteins to negatively regulate ABA signaling. The N-terminals of PP2Cs have a self-inhibition domain to repress their phosphatase activity, while EAR1 can bind to the N-terminal domain of PP2Cs and thus release this self-inhibition effect to enhance their phosphatase activity. Therefore, EAR1 can indirectly inhibit the kinase activity of SnRK2s and ABA signaling. Interestingly, we found that EAR1 protein was mainly localized on endoplasmic reticulum (ER) in the absence of ABA, whereas ABA treatment induced the translocation of EAR1 from ER to the nucleus. Together, our study identifies EAR1 as a new component of the ABA signaling pathway by interacting with and releasing the N-terminal self-inhibition of PP2Cs.
Next steps: We are exploring the molecular mechanisms regulating ABA-induced translocation of EAR1 from ER to the nucleus. In addition, we are also interested in elucidating the structural changes of PP2Cs in the presence of EAR1.
Kai Wang, Junna He, Yang Zhao, Ting Wu, Xiaofeng Zhou, Yanglin Ding, Lingyao Kong, Xiaoji Wang, Yu Wang, Jigang Li, Chun-Peng Song, Bao-Shan Wang, Shuhua Yang, Jian-Kang Zhu, and Zhizhong Gong. (2018). EAR1 negatively regulates ABA signaling by enhancing 2C protein phosphatase activity. Plant Cell Apr 2018, 30: 815-834; DOI: https://doi.org/10.1105/tpc.17.00875