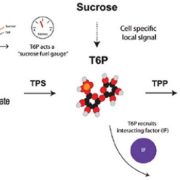
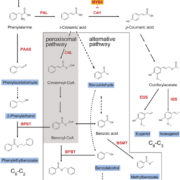
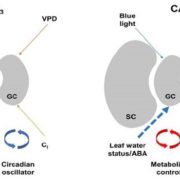
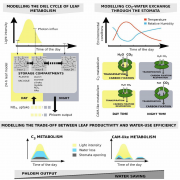
Plant RuBisCo assembly in E. coli with five chloroplast chaperones ($)
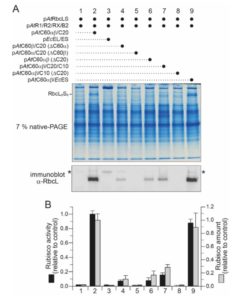 In plants, Ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCo), the enzyme responsible for fixing carbon, is a made up of 8 each of the large and small subunits, making the L8S8 form. Efforts to study this enzyme have been thwarted by the inability to assemble an active L8S8 form in a heterologous expression system such as E. coli. Assembly of the plant enzyme is complicated by the fact that the although the large subunit is translated in the chloroplast, the small subunit is translated in the cytosol and then imported into the plastid in unfolded form. Aigner, Wilson et al. have for the first time successfully expressed and assembled plant RuBisCo in E. coli, by co-expressing it with several chloroplast chaperones (proteins that can facilitate protein folding). This opens the door for important structure-function studies (Summary by Mary Williams). Science. 10.1126/science.aap9221
In plants, Ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCo), the enzyme responsible for fixing carbon, is a made up of 8 each of the large and small subunits, making the L8S8 form. Efforts to study this enzyme have been thwarted by the inability to assemble an active L8S8 form in a heterologous expression system such as E. coli. Assembly of the plant enzyme is complicated by the fact that the although the large subunit is translated in the chloroplast, the small subunit is translated in the cytosol and then imported into the plastid in unfolded form. Aigner, Wilson et al. have for the first time successfully expressed and assembled plant RuBisCo in E. coli, by co-expressing it with several chloroplast chaperones (proteins that can facilitate protein folding). This opens the door for important structure-function studies (Summary by Mary Williams). Science. 10.1126/science.aap9221