Engineering a faster Rubisco in tobacco chloroplasts
By Taiyu Chen and Lu-Ning Liu
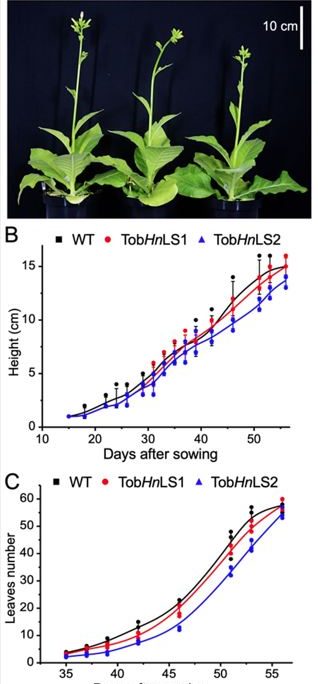
Figure legend: Engineering a Form IA Rubisco from the proteobacterium Halothiobacillus neapolitanus into tobacco chloroplasts could support growth of the transgenic tobacco lines (TobHnLS1 and TobHnLS2) in air complemented with 1% CO2, compared with WT (A). (B) Plant heights. (C), leaves numbers.
Background: Rubisco is the key enzyme responsible for fixing CO2. However, due to its intrinsically low catalytic turnover rate, Rubisco represents the ultimate rate-limiting step in plant photosynthesis. Improving Rubisco carboxylation and assembly in plants has been a long-standing challenge in crop engineering to meet the pressing need for increased global food production. There is mounting interest in replacing endogenous plant Rubisco with active non-native Rubisco candidates from other organisms to enhance photosynthetic carbon fixation.
Question: The folding and assembly of Rubisco in chloroplasts are intricate processes that usually require a series of ancillary factors. Seeking a new Rubisco variant that can be produced in chloroplasts with a high yield and high catalytic performance, without the requirement for cognate assembly factors and activases, could help improve carbon fixation in crop plants.
Finding: In this work, we introduced a Rubisco from a proteobacterium into tobacco chloroplasts to replace native tobacco Rubisco. In the proteobacteria, Rubisco is naturally encapsulated at a high density within a CO2-fixing protein organelle, the carboxysome. The foreign Rubisco derived from bacteria formed efficiently and was functional in chloroplasts without the need for exogenous chaperones. Intriguingly, the chloroplast-expressed bacterial Rubisco supported the autotrophic growth of transgenic plants at a similar rate to wild-type plants at 1% CO2.
Next Step: The successful production of functional bacterial Rubisco represents a step towards installing faster, highly active Rubisco, functional carboxysomes, and eventually active CO2-concentration mechanisms into chloroplasts to improve Rubisco carboxylation, with the intent of enhancing crop photosynthesis and crop yield on a global scale.
Reference:
Taiyu Chen, Saba Riaz, Philip Davey, Ziyu Zhao, Yaqi Sun, Gregory F. Dykes, Fei Zhou, James Hartwell, Tracy Lawson, Peter J. Nixon, Yongjun Lin, and Lu-Ning Liu. (2022). Producing fast and active Rubisco in tobacco to enhance photosynthesis. https://doi.org/10.1093/plcell/koac348


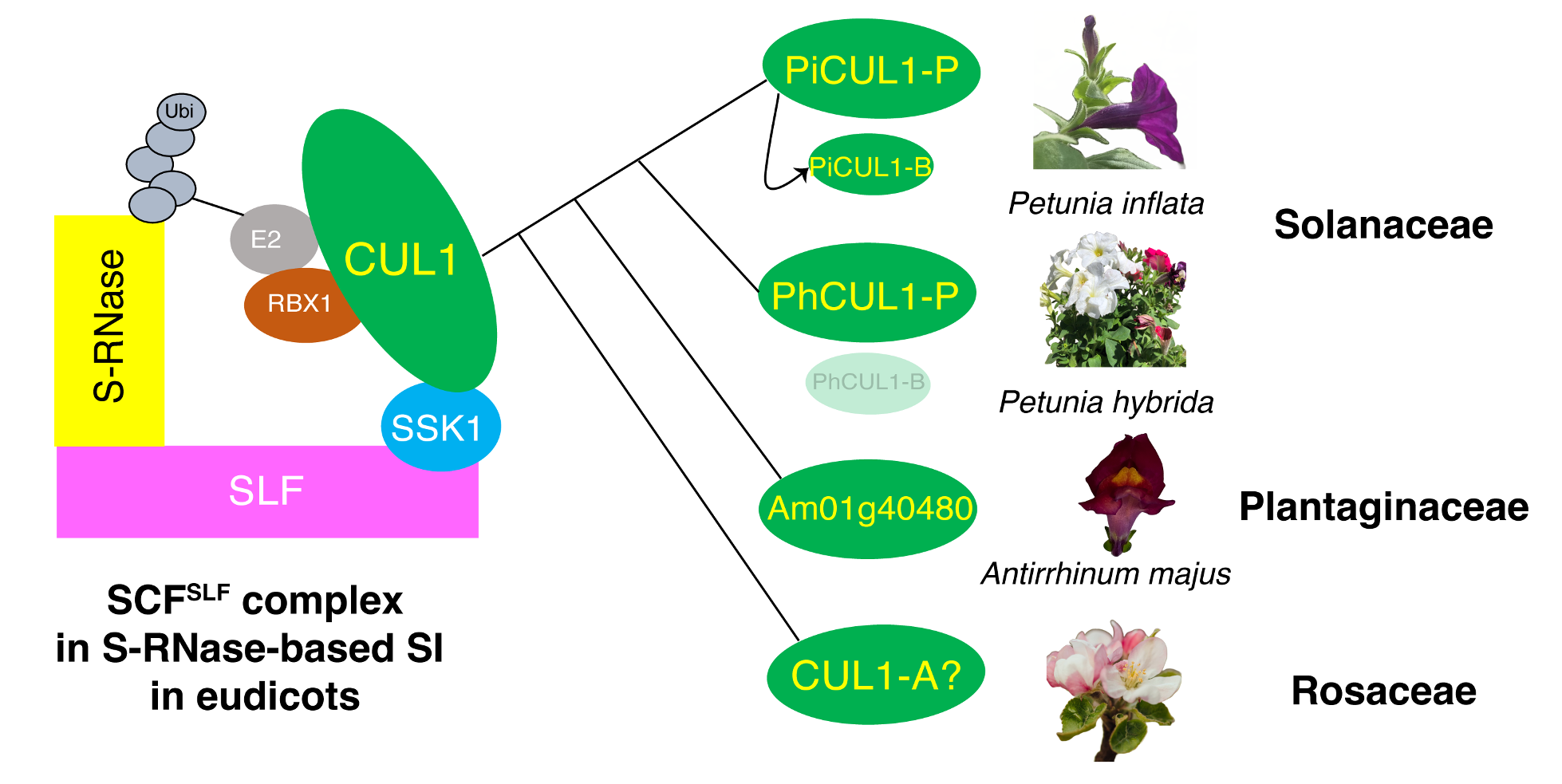 Findings: Using CRISPR/Cas9-mediated gene knockout, we found that, in Petunia inflata, pollen lacking functional PiCUL1-P could still detoxify nonself S-RNases during cross-compatible pollination, whereas pollen lacking both PiCUL1-P and another CUL1, PiCUL1-B, could not, suggesting functional redundancy of these two CUL1 proteins. In Petunia hybrida, we found a transposable element inserted in the promoter region of CUL1-B, which might result in drastic reduction of its transcript level, rendering PhCUL1-P essential for cross-compatibility. We did not find CUL1-B in the other Solanaceae species examined. Analyses of CUL1 evolution in eudicots revealed that all CUL1s involved, or potentially involved, in self-incompatibility possibly share a common ancestor.
Findings: Using CRISPR/Cas9-mediated gene knockout, we found that, in Petunia inflata, pollen lacking functional PiCUL1-P could still detoxify nonself S-RNases during cross-compatible pollination, whereas pollen lacking both PiCUL1-P and another CUL1, PiCUL1-B, could not, suggesting functional redundancy of these two CUL1 proteins. In Petunia hybrida, we found a transposable element inserted in the promoter region of CUL1-B, which might result in drastic reduction of its transcript level, rendering PhCUL1-P essential for cross-compatibility. We did not find CUL1-B in the other Solanaceae species examined. Analyses of CUL1 evolution in eudicots revealed that all CUL1s involved, or potentially involved, in self-incompatibility possibly share a common ancestor.
 Pablo González-Suárez, first author of
Pablo González-Suárez, first author of
 Kang Li, co-first author of
Kang Li, co-first author of  Peng Wang, co-first author of
Peng Wang, co-first author of  Longsheng Zhao, co-first author of “
Longsheng Zhao, co-first author of “