IN BRIEF by Jennifer Mach [email protected]
Green may have been the Pantone Color of the Year for 2013 (http://www.pantone.com/color-of-the-year-2013), but 2016 was a great year for papers on chlorophyll research, at The Plant Cell and beyond. In this year, we saw a pile of interesting papers examining chlorophyll degradation, including its regulatory mechanisms. For example, to identify transcription factors upstream of the key enzyme pheophorbide a oxygenase (PAO), which is a rate-limiting factor in chlorophyll breakdown, Ghandchi and co-authors (2016) used regression modeling and correlation analysis to look for factors that co-expressed with PAO under a variety of environmental conditions. They identified a correlation with specific transcription factors and a regulatory network that integrates stimuli from abiotic stresses (such as freezing and drought) and senescence to modulate specific transcription factors that control expression of PAO and other genes, and thus regulate chlorophyll breakdown. 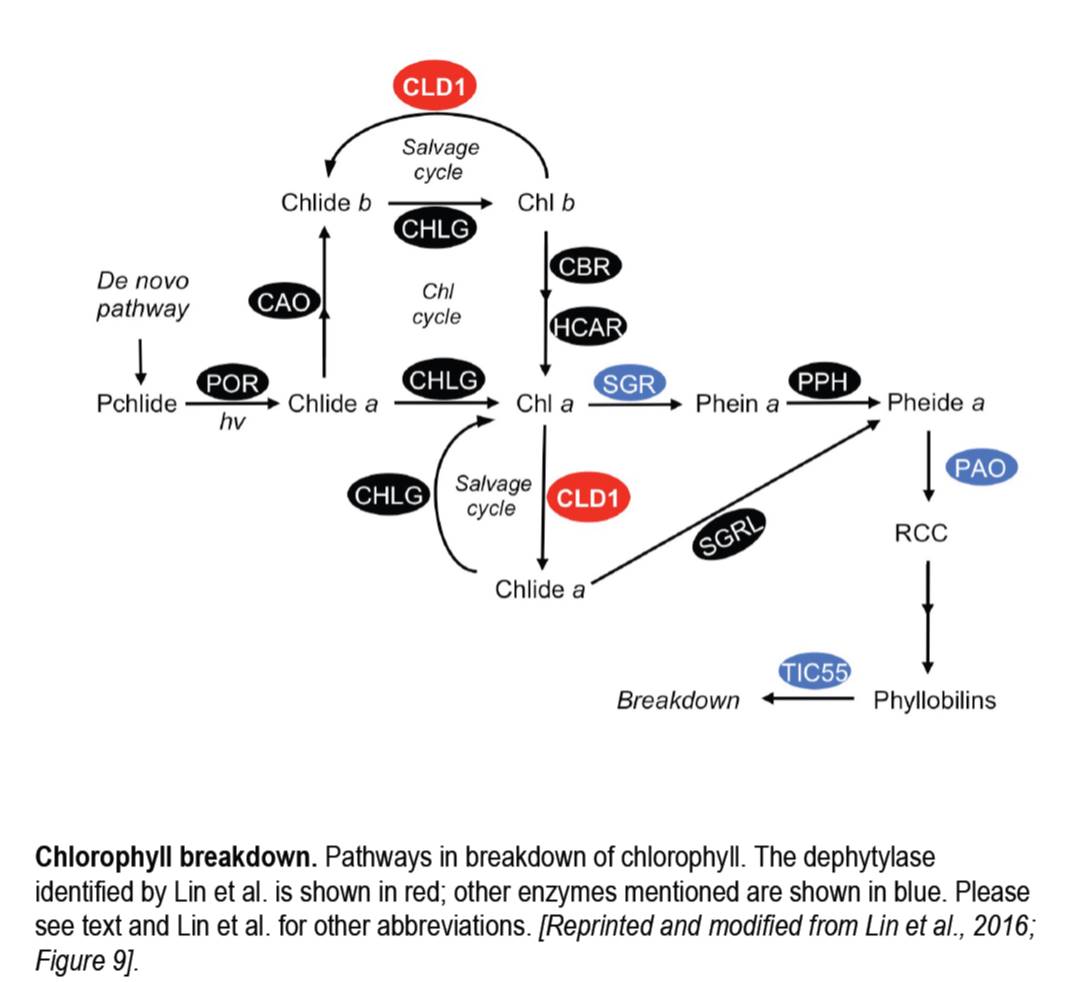
In another paper that generated some discussion, Wu and co-authors (2016) report that NON-YELLOWING2/STAY-GREEN2 (NYE2/SGR2), a close homolog of SGR/NYE1 (Mendel’s green cotyledon gene) functions as a positive regulator of chlorophyll breakdown and present several observations that are inconsistent with results from Sakuraba et al. (2014), specifically regarding the phenotypes of nye1 nye2 double mutants, which Wu et al. found to have a strong staygreen phenotype. These discrepancies, possibly due to growth conditions or genetic effects, and the role of NYE2/SGR2 await further clarification.
Enzymes also received a bit of attention in 2016, with the discovery of several missing links in the chlorophyll breakdown pathway (figure). For example, Shimoda et al. (2016) revealed that STAY-GREEN (SGR) functions as a magnesium dechelatase, which converts chlorophyll a to pheophytin a and also functions in degradation of the photosystems. Further along the chlorophyll breakdown pathway, Hauenstein and co-authors found an unexpected role for the chloroplast protein TRANSLOCON AT THE INNER CHLOROPLAST ENVELOPE 55 (TIC55) in the degradation of phyllobilins. TIC55 had been implicated in redox-regulated protein import in the chloroplast, making this finding surprising.
To cap off this year, in the December issue of The Plant Cell, Lin et al., (2016) report the exciting, somewhat serendipitous discovery of another missing enzyme, the activity that removes chlorophyll’s phytol tail during chlorophyll turnover. The authors started their research by examining a semidominant mutant that produces a heatsensitive phenotype and ended up identifying CHLOROPHYLL DEPHYTYLASE1 (CLD1). After cloning the locus responsible for the mutant phenotype, the authors speculated that the encoded enzyme might act on chlorophyll, based on its conservation and similarity to pheophytinase (PPH, see figure), particularly in the active site region. In vitro assays showed that CLD1 can dephytylate chlorophyll a and b, and pheophytin a, and that the mutant protein had higher activity than wild-type CLD1. Excess CLD1 activity caused mutant plants to accumulate chlorophyllides after heat shock and suppression of CLD1 by an artificial microRNA also caused plants to be sensitive to heat stress, with damage to Photosystem II, indicating the importance of chlorophyll turnover in photosystem stability.
Pantone might choose puce or magenta as the color of the year for 2017, but at The Plant Cell, we choose green every year.
Here’s to another great, green year!
REFERENCES
Ghandchi, F.P., Caetano-Anolles, G., Clough, S.J., and Ort, D.R. (2016). Investigating the Control of Chlorophyll Degradation by Genomic Correlation Mining. PLOS ONE 11(9): e0162327.
Hauenstein, M., Christ, B., Das, A., Aubry, S., and Hörtensteiner, S. (2016). A role for TIC55 as a hydroxylase of phyllobilins, the products of chlorophyll breakdown during plant senescence. Plant Cell 10.1105/tpc.16.00630.
Lin, Y.-P., Wu, M.-C., and Charng, Y.-y. (2016). Identification of Chlorophyll Dephytylase Involved in Chlorophyll Turnover in Arabidopsis. Plant Cell 10.1105/tpc.16.00478.
Sakuraba, Y., Park, S.Y., Kim, Y.S., Wang, S.H., Yoo, S.C., Hortensteiner, S., and Paek, N.C. (2014). Arabidopsis STAYGREEN2 is a negative regulator of chlorophyll degradation during leaf senescence. Mol. Plant 7:1288–1302.
Shimoda, Y., Ito, H., and Tanaka, A. (2016). Arabidopsis STAY-GREEN, Mendel’s green cotyledon gene, encodes magnesiumdechelatase. Plant Cell 28: 2147-2160.
Wu, S., Li, Z., Yang, L., Xie, Z., Chen, J., Zhang, W., Liu, T., Gao, S., Gao, J., Zhu, Y., Xin, J., Ren, G., and Benke Kuai, B. (2016) NON-YELLOWING2 (NYE2), a Close Paralog of NYE1, Plays a Positive Role in Chlorophyll Degradation in Arabidopsis. Molecular Plant 9: 624 – 627.
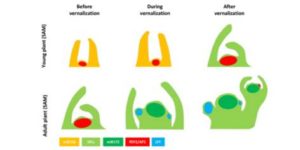 The transition between vegetative and reproductive stages in the plant life cycle implies a change in the developmental program of the shoot apical meristem to stop developing leaves and start developing floral buds. The factors that allow this transition to happen are many and the underlying mechanisms by which those factors induce flowering have been extensively studied. In this review, Hyun et al. go into the details of how miRNAs, especially miR156 and miR172, control when the plants get the competence to flower, and discuss their regulation of the SPL transcription factors and association with gibberellins. This miR156/SPL system seems to be ancient and quite conserved in flowering plants. (Summary by Gaby Auge) Plant Physiol. doi:10.1104/pp.16.01523
The transition between vegetative and reproductive stages in the plant life cycle implies a change in the developmental program of the shoot apical meristem to stop developing leaves and start developing floral buds. The factors that allow this transition to happen are many and the underlying mechanisms by which those factors induce flowering have been extensively studied. In this review, Hyun et al. go into the details of how miRNAs, especially miR156 and miR172, control when the plants get the competence to flower, and discuss their regulation of the SPL transcription factors and association with gibberellins. This miR156/SPL system seems to be ancient and quite conserved in flowering plants. (Summary by Gaby Auge) Plant Physiol. doi:10.1104/pp.16.01523 


 In Plant Physiology Preview you can get a head start on reading the excellent set of articles from a forthcoming special issue on Pollen Biology. Updates and research articles cover all aspects of this crucial part of reproductive biology, from the complex cell biology that underpins polar growth of pollen tubes to the signals exchanged between pistil and pollen that control mate selection. Selected reveiws cover Gametophytic pollen tube guidance (
In Plant Physiology Preview you can get a head start on reading the excellent set of articles from a forthcoming special issue on Pollen Biology. Updates and research articles cover all aspects of this crucial part of reproductive biology, from the complex cell biology that underpins polar growth of pollen tubes to the signals exchanged between pistil and pollen that control mate selection. Selected reveiws cover Gametophytic pollen tube guidance (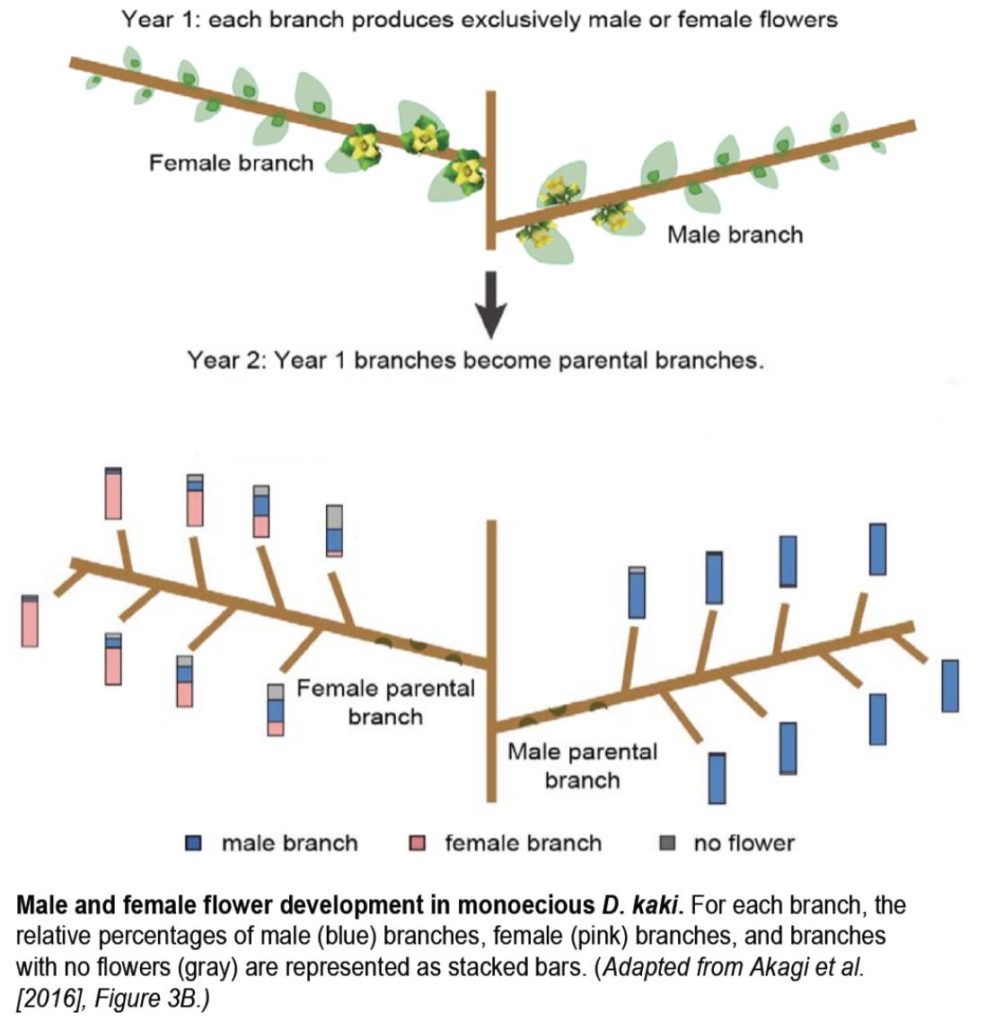 The position of a D. kaki bud on a branch affects the sex of the resulting flowers the following year (see figure). In June, each branch bears only male or female flowers and developing buds of unknown sex. The following year, the buds on these male or female parental branches develop into new, secondary branches, each bearing only male or female flowers. Apical buds on female branches almost always give rise to female secondary branches, whereas medial buds on the same branches have a nearly equal chance of forming male or female branches. By contrast, buds from male parental branches almost always develop into male branches, regardless of their position on the parental branch. This pattern suggests that an epigenetic mechanism is at play. Indeed, the methylation pattern across MeGI in D. kaki strongly mirrors this trend, with the highest methylation levels found in mature male flowers and buds and the lowest in their female counterparts. Therefore, gene silencing by methylation appears to regulate sex determination in D. kaki. This differential methylation pattern is much stronger in D. kaki than in D. lotus, which mainly uses OGI to silence this feminizing gene, suggesting that methylation of MeGI represents an alternative mechanism for sex determination in this autohexaploid persimmon species.
The position of a D. kaki bud on a branch affects the sex of the resulting flowers the following year (see figure). In June, each branch bears only male or female flowers and developing buds of unknown sex. The following year, the buds on these male or female parental branches develop into new, secondary branches, each bearing only male or female flowers. Apical buds on female branches almost always give rise to female secondary branches, whereas medial buds on the same branches have a nearly equal chance of forming male or female branches. By contrast, buds from male parental branches almost always develop into male branches, regardless of their position on the parental branch. This pattern suggests that an epigenetic mechanism is at play. Indeed, the methylation pattern across MeGI in D. kaki strongly mirrors this trend, with the highest methylation levels found in mature male flowers and buds and the lowest in their female counterparts. Therefore, gene silencing by methylation appears to regulate sex determination in D. kaki. This differential methylation pattern is much stronger in D. kaki than in D. lotus, which mainly uses OGI to silence this feminizing gene, suggesting that methylation of MeGI represents an alternative mechanism for sex determination in this autohexaploid persimmon species.