Luminaries: Elizabeth Van Volkenburgh
BY KEUM YOUNG LEE ASPB Student Ambassador, PhD Candidate in the School of Environmental and Forest Sciences, University of Washington (Originally published January 2014)
Professor, University of Washington
 Elizabeth Van Volkenburgh has been a faculty member in the Botany and Biology Departments, with an adjunct appointment in the School of Environmental and Forest Sciences, at the University of Washington since 1987. She also served as divisional dean for research, College of Arts and Sciences (2004–2006).
Elizabeth Van Volkenburgh has been a faculty member in the Botany and Biology Departments, with an adjunct appointment in the School of Environmental and Forest Sciences, at the University of Washington since 1987. She also served as divisional dean for research, College of Arts and Sciences (2004–2006).
Elizabeth has a long history as an expert in the field of plant physiology. After receiving her BS in botany in 1973 from Duke University, she went on to get her PhD in plant physiology at the University of Washington, where she focused on leaf growth response to light. Prior to entering graduate school, she worked as a technician at the Smithsonian Institution Botany Department and at the Duke University Phytotron. She did postdoctoral work at the University of Illinois at Champaign–Urbana, the University of Lancaster in England, and the University of Washington and eventually found her way to a faculty position in the Botany and Biology Departments at the University of Washington. On a beautiful sunny day in Seattle, she was kind enough to take time out of her hectic schedule to answer a few questions about her many projects designed to discover how plants respond and adapt to their environment.
You received your BS in botany in 1973. If you look back, do you remember what made you choose botany as your major? What made you go into plant science?
When I was an undergraduate, I tried very hard to major in economics and political science. That’s because my mother encouraged me to become a lawyer. I thought that those were subjects to learn to become a lawyer, but I didn’t do very well because I didn’t understand the content. I had done very well in biology in high school, and also my freshman year in college I took biology because I knew I would do well. So by the end of my second year in college, I had some biology courses where I had good grades and the other courses where I had not-so-good grades, so I switched. I chose plants in part to avoid the premedical students, who were so intense and very competitive.
You are most interested in the relationship between plants and their environment. In your opinion, why is asking how plants “behave” in response to their environment so important?
My interest in plants is mostly in how they work. Maybe the best way to find out how they work (or how anything else works) is to perturb them, to give them a problem to solve. I am really interested in growth and regulation of growth rate, but if you stress a plant, it might grow slower, or maybe grow better, having solved the problem of the stress, so then the stress or the environmental signal is a way of perturbing the system to ask what’s going on underneath. That’s probably why I do it. In between college and graduate school, I worked as a lab technician for one year. Dr. Paul Kramer’s group was working on drought stress. That’s probably how I was introduced to the idea of looking at how plants adapt to drought. Even though I am interested in basic cell physiology, I have always been treating my plants to one type of stress or another just to test them. In my PhD work, I was interested in knowing what regulates leaf expansion. One way to ask was to use light as a tool, because it was known that light would make leaves grow faster. We could compare slower growth in the dark and faster growth in the light to find out how growth rate is regulated.
What’s your central focus on plant behaviors regulated by various environmental factors? (What do you measure to discover the mechanisms, and why?)
My central focus is always growth rate, and growth always means cell expansion. It may also involve cell division, but I am not studying the process of cell division or mitosis, only the increase in volume of cells, so cell expansion. We measure the size of the plant with a ruler or microscope or sometimes by weighing with a balance. Often we are working on whole leaves or excised pieces of leaf and sometimes single leaf cells. We measure their size, either their dimensions or their weights, and then we also measure the physiological processes likely to be regulating their change in size. Early on, I was interested in cell wall extensibility. Now I am more interested in ion flux across the plasma membrane. For this we use pH electrode, microelectrode, or other electrophysiological methods.
Regarding leaf growth physiology responding to a variety of environmental cues, what are some of the highlights at this point?
For me the highlights are always the biggest unanswered questions. I would answer you by saying what are the biggest mysteries that attract me rather than what the most recent discoveries are. We know a little bit about membrane transport in a growing leaf cell involving the proton pump and potassium ion channels and probably also sucrose transporters. Just focusing on those three transporters, the questions that puzzle me are how light, specifically blue light or red light, interacts with those transporters and whether or not the hormone auxin is in some way involved in the growth rate control of leaf cells. The role of auxin seems to be debated, but I say “yes.” I am approaching the question slowly with auxin since the literature is conflicted. Some evidence exists for the role of auxin and growing leaves, and other evidence says either auxin is inhibitory or has no role at all. I am reading the literature first.
Originally my idea was to use different wavelengths of light as handles to get at some molecules or biochemical processes that regulate cell growth, and we got close, but what we don’t know, for example, is whether or not blue light is perceived by chlorophyll, cryptochrome, or phototropin. If we guess phototropin, we don’t know what phototropin is interacting with: proton pump, potassium channel, or auxin transport. If we say phototropin is working with auxin transport, we run into the problem that nobody knows whether or not auxin is really playing a role in leaf cell growth. There are a number of things to test and untangle. The reason I want to know more generally is that the plant has to make its leaves in order to collect enough carbon to feed seed production. Whatever is limiting leaf expansion ultimately limits yield of the plant. So it turns out that leaves are highly integrated; they use probably every signaling system in the plant to tell them how fast to grow and end up being complicated.
Part of your research deals with light perception by two photoreceptor systems (one being the family of phytochromes and the other being the yet uncharacterized family of blue light photoreceptors). Can you briefly explain the importance of this research and any breakthroughs you may have had?
I haven’t actually been working on that for a number of years, so there aren’t any recent breakthroughs from me. There has been work by other people focusing on phototropin in leaf growth, but the results and the conclusions for me confuse phototropin with photosynthetic output. When I started thinking about blue light stimulating leaf expansion, we used seedlings that were still partially etiolated and thus relying on photoreceptors more. Another thing that is important to do, and we’ve done, is to show that these phytochromes and blue light photoreceptors are operating normally in green leaves beyond the seedling stage. Then they are helping the leaf reach an appropriate size at the same time as chlorophyll is absorbing light for photosynthesis. The connection between those three kinds of photoreceptors is interesting to me.
There is another aspect to work that we’ve done more recently, which is considering how leaves maintain themselves as flat structures rather than curling under in epinasty or curling up in hyponasty. There is some evidence showing auxin and ethylene are involved in the process. We have evidence that auxin is involved even without ethylene. There is other work that shows if we remove the phototropin from Arabidopsis, leaves are curled, so the phototropin probably has something to do with the maintenance of flat structure. It could be, although I haven’t tested it directly, that the maintenance of flat structure will contribute to the rate of growth, and the leaf will grow faster if the mechanism for keeping it flat isn’t compromised. If whatever keeps it flat is broken, so that it is curling, then the rate of expansion would be reduced as well. With Bob Cleland and Rainer Stahlberg, we are working on the maintenance of flat structure in leaves, and that isn’t published yet.
Your “microevolution” study using corn addressed the physiological basis of high yield in newer corn hybrids. Would you explain the mechanisms by which newer varieties withstand density stress?
My collaborator, E. David Ford, and I looked at corn plants that were developed over 70 or 80 years. As corn breeders selected high-yielding lines of corn, they improved the corn steadily over time. We compared the different lines to see what was the characteristic of the modern corn compared to the older lines. There were two major findings. It had already been recognized that the modern corn hybrids hold their leaves more erect, so they don’t shade each other. When these varieties are planted very densely, each plant occupies less footprint, so the plant shades itself and shades its neighbors less. We wanted to know a little bit about how the plant holds its leaves up. We did some biomechanical studies to look at strength of the leaves and so on. For wild plants, also older corn plants, if they detect far red light as they would if they detect their neighbor, they respond by holding their leaves upright. The modern hybrids will also move their leaves a little bit from less erect to more erect, but the modern hybrids are hard-wired to have their leaves more erect to begin with. So they don’t shade themselves as much. That gave us the idea that something was broken in the modern hybrids. They were less flexible, less responsive to the environment. Perhaps we could consider them to be hard-wired for stress tolerance. We started looking at seedlings and seedling growth, which we understood a little bit better. With Martin Fellner, we investigated the effect of light on elongation of seedlings and found that in the modern hybrids they are less sensitive to light because they were also less sensitive to auxin. It turned out when Martin looked at the expression of auxin binding proteins, there was a difference between the modern hybrids and older hybrids. It suggests that in the wild, plant phytochrome interacts with auxin signaling and that causes leaves to grow more upright, but in the modern hybrids the light can interact with auxin signaling, except the auxin signaling pathway itself is different and it doesn’t change the morphology of the leaves. The morphology of the leaves is one thing, but another thing that happens to plants when they are crowded and they sense neighbors is that they stop reproducing. It seems that the modern hybrids in corn, because their signaling with auxin is altered, don’t down-regulate reproduction, so they go ahead and make the ears even when crowded.
You are also looking for the biomechanics of free-coiling in cucumber. How are you addressing this issue? What is the physiological and perhaps developmental basis for tissue changes that lead to coiling?
This is a really fun project. It started when Annika Eberle came to the University of Washington to work as a graduate student in mechanical engineering, and she asked me to coadvise her with Professor Minoru Taya. Since they are mechanical engineers, they don’t have a lot of education or much intuition about the physiology of the plants. We were really lucky to have a biology undergraduate, Chris Stripinis, who came in to work with us. Chris ended up integrating the biology from me and the engineering from the engineers and tried to assemble ideas in his own thinking. It was wonderful to watch. The only way we could have group meetings was with a fake tendril, a swim noodle. Then we could make this thing turn and imagine the cells that were inside if it were really a tendril, and figure out where the stresses are, what caused it to bend and move, and what prevented bending. That was how we communicated between the biology and engineering. My thinking of the tendril was that it was growing like a stem, and I kind of know how stems grow compared to how leaf grows. In a way it is similar to the question of how a leaf grows flat. We think a reason a leaf is flat is because both of the epidermal layers grow at the same rate, but if the upper epidermal cells grow faster than lower, it’s going to be curved. If the vein grows faster than the leaves, it is going to tear, and if the vein grows slower than the leaves, it is going to wrinkle. In the same way, we can think about the tendril. It is going to coil if one side grows faster than the other side. You can imagine it’s sort of like phototropism where auxin is transported laterally. The engineers pointed out to me that that would cause a circle, but it won’t spiral, and in order to have a spiral, there has to be something asymmetric inside that keeps the whole thing from landing on itself and instead making a helix. We don’t know how the tendril does it. The literature we found suggested that the coil was related to one layer of cells, which then becomes lignified, but we found another paper and also our own work that shows that the coil happens first and then lignification happens later. Perhaps stem circumnutation is a good model. Probably the coil of tendril has a flow of auxin from the tip down the tendril, but it’s not coming straight down the tendril—it’s coming in a twist. When we looked down the surface of the tendril, we found that the epidermal cells actually accomplished twist, and that would be asymmetry, but we are not yet in agreement between the engineers and the biologists over whether or not that’s enough of a twist. It would be fabulous to visualize auxin transport inside the organ.
Any final words you’d like to leave us with?
I really enjoy investigating plants, and I like designing experiments. This is an activity that so many people could do and would find fun to do if they were given access through their biology education. Sometimes it’s not the “A” student who is the best designer of experiments. I wish we could attract a wider range of question-askers, because questions are there and they need to be approached with different methods and take different kinds of models and characters to do it. It can be a lot of fun—not always a high-stress activity. My final words would be to encourage all comers to try out the fun of asking how plants behave.
SOURCE: Lee, K.Y. (2013) Luminaries: Elizabeth Van Volkenburgh. ASPB News 41(1): 13 – 15. Reprinted by permission from ASPB.


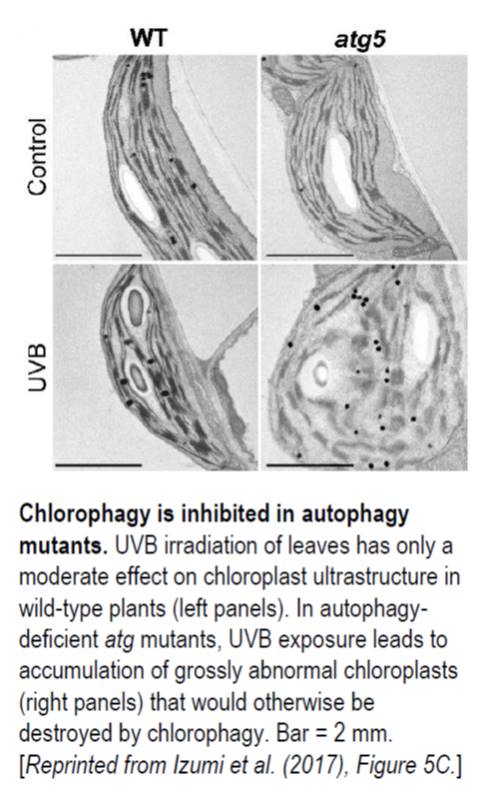 Autophagy, or “self eating,” is the process cells use to consume unwanted intracellular structures such as damaged organelles, excess membranes, and unneeded cellular proteins (
Autophagy, or “self eating,” is the process cells use to consume unwanted intracellular structures such as damaged organelles, excess membranes, and unneeded cellular proteins (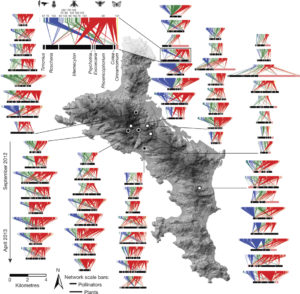 How does ecosystem restoration affect ecosystem services such as pollination? Kaiser-Bunbury et al. analysed 64 plant-pollinator networks across four restored and four unrestored communities. Restoration involved the removal of all exotic plants (nearly 40,000 individuals). After restoration, over a period of several months, they recorded pollinator-plant interactions: a total of more than 12,000 pollinator visits. They found on average 20% more pollinator species in the restored compared to the unrestored communities. The greater pollination networks indicate greater functional redundancy and lower mutual dependency, leading to more efficient pollination and ecosystem resilience. Nature
How does ecosystem restoration affect ecosystem services such as pollination? Kaiser-Bunbury et al. analysed 64 plant-pollinator networks across four restored and four unrestored communities. Restoration involved the removal of all exotic plants (nearly 40,000 individuals). After restoration, over a period of several months, they recorded pollinator-plant interactions: a total of more than 12,000 pollinator visits. They found on average 20% more pollinator species in the restored compared to the unrestored communities. The greater pollination networks indicate greater functional redundancy and lower mutual dependency, leading to more efficient pollination and ecosystem resilience. Nature 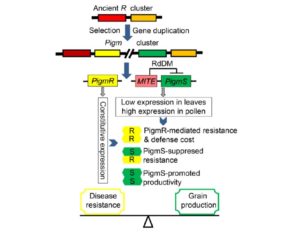 The indigenous Chinese rice variety Gumei 4 (GM4) shows durable and specific resistance to the rice blast fungal pathogen Manaporthe oryzae. Deng et al. mapped and sequenced the resistance locus Pigm, and found that it contains a cluster of 13 NLR (nucleotide-binding leucine-rich repeat) genes, three of which appear to be transcribed. One of these, PigmR, was sufficient to confer resistance. By contrast, ectopic expression of PigmS promotes fungal infection in plants carrying PigmR. Using a yeast two-hybrid assay, the authors showed that that PigmS and PigmR form a heterodimer that impairs functional PigmR homodimer function and resistance. Furthermore, PigmS expression is epigenetically regulated and shows low expression in leaves (where it attenuates resistance) and high expression in pollen, where it appears to contribute to seed set and therefore yield. As the authors conclude, they have identified “two NLR genes which have evolved opposing function in disease resistance and yield in a single cluster”. Science
The indigenous Chinese rice variety Gumei 4 (GM4) shows durable and specific resistance to the rice blast fungal pathogen Manaporthe oryzae. Deng et al. mapped and sequenced the resistance locus Pigm, and found that it contains a cluster of 13 NLR (nucleotide-binding leucine-rich repeat) genes, three of which appear to be transcribed. One of these, PigmR, was sufficient to confer resistance. By contrast, ectopic expression of PigmS promotes fungal infection in plants carrying PigmR. Using a yeast two-hybrid assay, the authors showed that that PigmS and PigmR form a heterodimer that impairs functional PigmR homodimer function and resistance. Furthermore, PigmS expression is epigenetically regulated and shows low expression in leaves (where it attenuates resistance) and high expression in pollen, where it appears to contribute to seed set and therefore yield. As the authors conclude, they have identified “two NLR genes which have evolved opposing function in disease resistance and yield in a single cluster”. Science 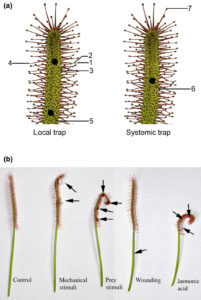 Without eyes, mouths or noses, how do carnivorous plants know that they’ve captured prey? Previous studies in various carnivorous species have shown that electrical signals as well as the jasmonate defense hormones contribute to prey detection. Krausko et al. examined these signals in leaves of the sundew Drosera capensis, which has leaves covered with sticky tentacles that roll inwards to trap prey. The authors identified a sequential set of events upon prey perception, starting with a steep electrical signal that initiates in the tentacles; mechanical wounding alone is sufficient for this reaction. Only when prey is present, this initial response is followed by membrane oscillations and also jasmonate accumulation at the site of prey capture, leading to strong bending and high levels of digestive enzyme production. Inorganic salts and chitin may act as chemical signals of prey. New Phytol.
Without eyes, mouths or noses, how do carnivorous plants know that they’ve captured prey? Previous studies in various carnivorous species have shown that electrical signals as well as the jasmonate defense hormones contribute to prey detection. Krausko et al. examined these signals in leaves of the sundew Drosera capensis, which has leaves covered with sticky tentacles that roll inwards to trap prey. The authors identified a sequential set of events upon prey perception, starting with a steep electrical signal that initiates in the tentacles; mechanical wounding alone is sufficient for this reaction. Only when prey is present, this initial response is followed by membrane oscillations and also jasmonate accumulation at the site of prey capture, leading to strong bending and high levels of digestive enzyme production. Inorganic salts and chitin may act as chemical signals of prey. New Phytol. 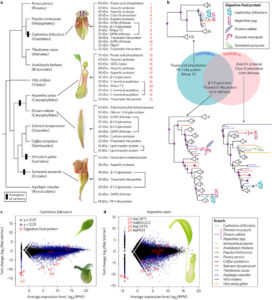 Cephalotus follicularis is a heterophyllous pitcher plant that makes two types of leaves, carnivorous and non-carnivorous. By growing plants at different temperatures, Fukushima et al. were able to get plants to produce one of the two leaf forms. They sequenced the plant’s genome and compared transcriptomes between the two types of leaves. Carnivory-specific (or enriched) genes include those involved in the production of nectar, slippery waxes and digestive fluids. The authors showed that a few digestive enzymes including a chitinase, a purple acid phosphatase, and an RNase, show a convergent pattern of amino acid substitutions amongst carnivorous species. They interpret this convergent evolution as evidence that “there are few available evolutionary pathways for angiosperms to become carnivorous.” Nature Ecol. Evol.
Cephalotus follicularis is a heterophyllous pitcher plant that makes two types of leaves, carnivorous and non-carnivorous. By growing plants at different temperatures, Fukushima et al. were able to get plants to produce one of the two leaf forms. They sequenced the plant’s genome and compared transcriptomes between the two types of leaves. Carnivory-specific (or enriched) genes include those involved in the production of nectar, slippery waxes and digestive fluids. The authors showed that a few digestive enzymes including a chitinase, a purple acid phosphatase, and an RNase, show a convergent pattern of amino acid substitutions amongst carnivorous species. They interpret this convergent evolution as evidence that “there are few available evolutionary pathways for angiosperms to become carnivorous.” Nature Ecol. Evol. 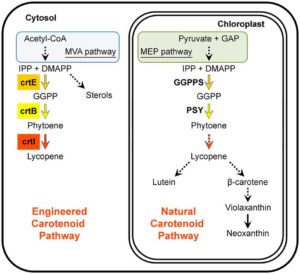 A healthy human diet should include phytonutrients such as carotenoids. Several approaches including classical breeding and transgenic plant production have been used to increase carotenoid abundance in plant tissues; challenges to these approaches include feedback controls, cell toxicity due to abnormally high compound levels, and compartmentalization of enzymes and intermediates. Majer et al. demonstrate high levels of lycopene production in leaf cytosolic tissues by way of a transiently expressed viral vector carrying three enzymes derived from bacteria. They report an unexpected tissue yellowing in cells expressing just the first enzyme of the pathway, which should accumulate a colorless intermediate; this yellowing they ascribe to chlorosis and decreased accumulation of chlorophyll through an as yet unexplained mechanism. However, this effect can be harnessed as a visual marker to track virus dynamics (stability, replication and movement). Sci. Reports
A healthy human diet should include phytonutrients such as carotenoids. Several approaches including classical breeding and transgenic plant production have been used to increase carotenoid abundance in plant tissues; challenges to these approaches include feedback controls, cell toxicity due to abnormally high compound levels, and compartmentalization of enzymes and intermediates. Majer et al. demonstrate high levels of lycopene production in leaf cytosolic tissues by way of a transiently expressed viral vector carrying three enzymes derived from bacteria. They report an unexpected tissue yellowing in cells expressing just the first enzyme of the pathway, which should accumulate a colorless intermediate; this yellowing they ascribe to chlorosis and decreased accumulation of chlorophyll through an as yet unexplained mechanism. However, this effect can be harnessed as a visual marker to track virus dynamics (stability, replication and movement). Sci. Reports  There are more than 25,000 species in the Orchidaceae, the orchid family. Chao et al. have updated and restructured the Orchidstra database, which now houses more than half-a million protein-coding genes from 18 species (12 genera and five subfamilies). Access and explore it at
There are more than 25,000 species in the Orchidaceae, the orchid family. Chao et al. have updated and restructured the Orchidstra database, which now houses more than half-a million protein-coding genes from 18 species (12 genera and five subfamilies). Access and explore it at 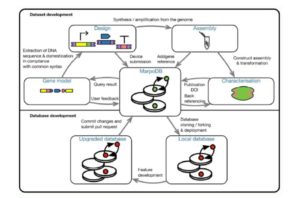 Marchantia polymorpha (a liverwort) is a living relative of the earliest terrestrial plants. As it has a simple genome and morphology and is readily transformable, it provides a good platform for synthetic biology (see
Marchantia polymorpha (a liverwort) is a living relative of the earliest terrestrial plants. As it has a simple genome and morphology and is readily transformable, it provides a good platform for synthetic biology (see