Rapid cytosolic calcium elevations in Arabidopsis during aphid feeding
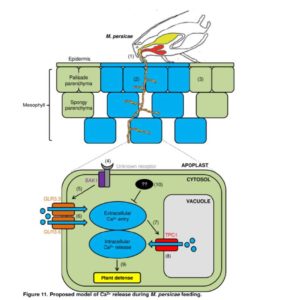 Calcium signaling is a common plant response to many different stimuli. Vincent et al. used a fluorescent calcium reporter, GCaMP3, to record calcium responses in Arabidopsis to feeding by aphids (specifically, the green peach aphid Myzus persicae). Through analysis of various mutants, key components of the aphid calcium response were identified and a model proposed. The response is abolished in mutants of the co-receptor BAK1, and also in mutants of the plasma membrane cation-permeable channels GLR3.3 and GLR3.6. The calcium-permeable vacuolar channel TWO-PORE CHANNEL 1 (TPC1) also has a role, with the signal attenuated in loss-of-function mutants, and systemically enhanced (with a concommitant loss of aphid fecundity) in over-activation lines. These results demonstrate the importance of calcium signaling in plant responses to aphids, and a potential strategy for insect-resistance. Plant Cell 10.1105/tpc.17.00136
Calcium signaling is a common plant response to many different stimuli. Vincent et al. used a fluorescent calcium reporter, GCaMP3, to record calcium responses in Arabidopsis to feeding by aphids (specifically, the green peach aphid Myzus persicae). Through analysis of various mutants, key components of the aphid calcium response were identified and a model proposed. The response is abolished in mutants of the co-receptor BAK1, and also in mutants of the plasma membrane cation-permeable channels GLR3.3 and GLR3.6. The calcium-permeable vacuolar channel TWO-PORE CHANNEL 1 (TPC1) also has a role, with the signal attenuated in loss-of-function mutants, and systemically enhanced (with a concommitant loss of aphid fecundity) in over-activation lines. These results demonstrate the importance of calcium signaling in plant responses to aphids, and a potential strategy for insect-resistance. Plant Cell 10.1105/tpc.17.00136


 Anyone who is trying to manage a laboratory on a budget will enjoy reading about the efforts of Henrici et al., who have designed a pair of plasmids that together can be digested with one of two restriction enzymes to produce either 100-bp or 1000-bp DNA molecular weight markers. As the authors report, “We would like to offer the pPSU1 & pPSU2 pair of plasmids as an alternative to commercial or other homemade sources of DNA ladders. We therefore make available the pPSU1 and pPSU2 plasmids to the nonprofit academic community without licensing requirements. The pPSU1 and pPSU2 plasmids are being deposited in the Addgene and DNASU repositories to facilitate distribution.” Sci. Reports
Anyone who is trying to manage a laboratory on a budget will enjoy reading about the efforts of Henrici et al., who have designed a pair of plasmids that together can be digested with one of two restriction enzymes to produce either 100-bp or 1000-bp DNA molecular weight markers. As the authors report, “We would like to offer the pPSU1 & pPSU2 pair of plasmids as an alternative to commercial or other homemade sources of DNA ladders. We therefore make available the pPSU1 and pPSU2 plasmids to the nonprofit academic community without licensing requirements. The pPSU1 and pPSU2 plasmids are being deposited in the Addgene and DNASU repositories to facilitate distribution.” Sci. Reports 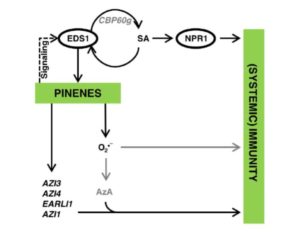 Pathogen perception leads to local and systemic immune responses including systemic acquired resistance (SAR). The nature of the mobile signals and their movements remain uncertain. Riedlmeier et al. demonstrated that certain monoterpenes including α- and β-pinene accumulate in SAR-inducing conditions and enhance systemic resistance. Futher, they show evidence for “a tight association between plant-to-plant SAR-like signaling and monoterpene emissions.” Plant Cell
Pathogen perception leads to local and systemic immune responses including systemic acquired resistance (SAR). The nature of the mobile signals and their movements remain uncertain. Riedlmeier et al. demonstrated that certain monoterpenes including α- and β-pinene accumulate in SAR-inducing conditions and enhance systemic resistance. Futher, they show evidence for “a tight association between plant-to-plant SAR-like signaling and monoterpene emissions.” Plant Cell 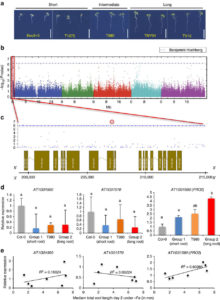 Iron is an essential nutrient that plants assimilate from the soil. Moderate iron deficiency induces an increase in primary root length and lateral root production. Satbhai et al. examined natural variation of root responses and showed a correlation between root length and allelic variation at the FRO2 locus which encodes a membrane-bound ferric chelate reductase involved in the reduction of Fe3+ to Fe2+ prior to uptake into the root. Specifically, elevated expression levels of FRO2 are correlated with longer roots in low iron conditions. Nature Comms.
Iron is an essential nutrient that plants assimilate from the soil. Moderate iron deficiency induces an increase in primary root length and lateral root production. Satbhai et al. examined natural variation of root responses and showed a correlation between root length and allelic variation at the FRO2 locus which encodes a membrane-bound ferric chelate reductase involved in the reduction of Fe3+ to Fe2+ prior to uptake into the root. Specifically, elevated expression levels of FRO2 are correlated with longer roots in low iron conditions. Nature Comms. 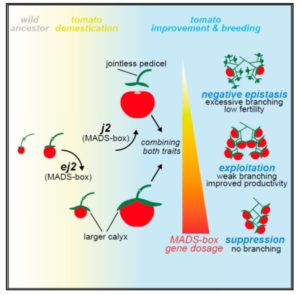 Tomatoes normally grow as multiple flowers along a single branch. Soyk et al. explored a large collection of wild and domesticated accessions to identify those with branched inflorescences, which should be able to produce more fruit per plant. They identified a few related branchy mutants which they called s2, which is highly branching but with low fertility so fails to form many fruit. They subsequently recognized that the s2 phenotype arises from variation in two unlinked genes, J2 and EJ2, both of which encode MADS box transcription factors. The ej2w allele increases fruit size, and was probably selected for during domestication. The j2TE allele provides a desirable jointless phenotype that prevents fruit from dropping, but when combined with ej2w leads to excessive branching and decreased fruit yield. By separating out these two loci and engineering new alleles, tomatoes with more but not too much more branching and higher yelds were produced. Cell
Tomatoes normally grow as multiple flowers along a single branch. Soyk et al. explored a large collection of wild and domesticated accessions to identify those with branched inflorescences, which should be able to produce more fruit per plant. They identified a few related branchy mutants which they called s2, which is highly branching but with low fertility so fails to form many fruit. They subsequently recognized that the s2 phenotype arises from variation in two unlinked genes, J2 and EJ2, both of which encode MADS box transcription factors. The ej2w allele increases fruit size, and was probably selected for during domestication. The j2TE allele provides a desirable jointless phenotype that prevents fruit from dropping, but when combined with ej2w leads to excessive branching and decreased fruit yield. By separating out these two loci and engineering new alleles, tomatoes with more but not too much more branching and higher yelds were produced. Cell 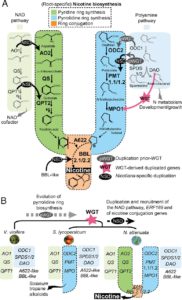 The genus Nicotiana encompasses several species and hybrids, the most famous being Nicotiana tabacum, cultivated for production of tobacco. Xu et al. sequenced the genome of Nicotiana attenuata and Nicotiana obtusifolia with an interest in identifying the origins of nicotine biosynthesis. Nicotine is a toxic alkaloid produced to protect against herbivory, but also the addictive compound in tobacco products. Analysis of these genomes revealed that nicotine synthesis evolved in Nicotiana from duplication in the polyamine and nicotinamide adenine dinucleotide pathways, as well as proliferation of transposable elements. Proc. Natl. Acad. Sci. USA
The genus Nicotiana encompasses several species and hybrids, the most famous being Nicotiana tabacum, cultivated for production of tobacco. Xu et al. sequenced the genome of Nicotiana attenuata and Nicotiana obtusifolia with an interest in identifying the origins of nicotine biosynthesis. Nicotine is a toxic alkaloid produced to protect against herbivory, but also the addictive compound in tobacco products. Analysis of these genomes revealed that nicotine synthesis evolved in Nicotiana from duplication in the polyamine and nicotinamide adenine dinucleotide pathways, as well as proliferation of transposable elements. Proc. Natl. Acad. Sci. USA 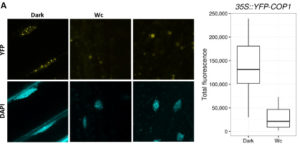 SPA (SUPPRESSOR OF PHYTOCHROME A-105) proteins form a complex with COP1 (CONSTITUTIVELY PHOTOMORPHOGENIC 1) and are required for COP1 to respond to changes in the light environment and transduce light signals and influence plant development. In this paper, Balcerowicz et al. investigated the importance of SPA proteins in the COP1/SPA complex. Using fluorescence, they could locate exactly where COP1 was in seedlings grown in light or kept in darkness, in a background with or without SPA proteins. Their results show that SPA proteins are not necessary for COP1 to localize in the nucleus but they are required for the complex to leave the nucleus after exposure to light, without affecting its degradation rate. Furthermore, they show that COP1 localization mediated by SPA proteins respond differently to different light wavelengths. Altogether, the results show that SPA proteins are necessary for COP1 responsiveness to changes in the light environment by altering its subcellular localization and, in consequence, the proper response of plants to light. (Review by
SPA (SUPPRESSOR OF PHYTOCHROME A-105) proteins form a complex with COP1 (CONSTITUTIVELY PHOTOMORPHOGENIC 1) and are required for COP1 to respond to changes in the light environment and transduce light signals and influence plant development. In this paper, Balcerowicz et al. investigated the importance of SPA proteins in the COP1/SPA complex. Using fluorescence, they could locate exactly where COP1 was in seedlings grown in light or kept in darkness, in a background with or without SPA proteins. Their results show that SPA proteins are not necessary for COP1 to localize in the nucleus but they are required for the complex to leave the nucleus after exposure to light, without affecting its degradation rate. Furthermore, they show that COP1 localization mediated by SPA proteins respond differently to different light wavelengths. Altogether, the results show that SPA proteins are necessary for COP1 responsiveness to changes in the light environment by altering its subcellular localization and, in consequence, the proper response of plants to light. (Review by 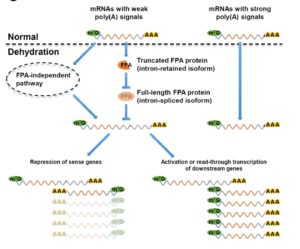 Sun et al. observed that under dehydration stress, many genes showed a 3′ untranslated region (3′ UTR) extension of roughly 200 – 800 nucleotides. The outcome of these extensions appears to be the regulation of other genes. For example, through their extension, many (more than 600) of the extended mRNAs overlapped with genes on the opposite strand (described here as sense transcripts), and many of these sense transcript genes showed a decrease in expression levels. Other extended RNAs overlapped with promoters of genes on the same strand via their extension, resulting in an increase in expression levels of the overlapped genes. This report suggests a novel strategy for stress-regulated gene expression. Genome Research
Sun et al. observed that under dehydration stress, many genes showed a 3′ untranslated region (3′ UTR) extension of roughly 200 – 800 nucleotides. The outcome of these extensions appears to be the regulation of other genes. For example, through their extension, many (more than 600) of the extended mRNAs overlapped with genes on the opposite strand (described here as sense transcripts), and many of these sense transcript genes showed a decrease in expression levels. Other extended RNAs overlapped with promoters of genes on the same strand via their extension, resulting in an increase in expression levels of the overlapped genes. This report suggests a novel strategy for stress-regulated gene expression. Genome Research 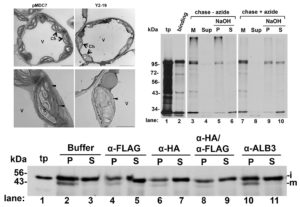 Chloroplasts have evolved from photosynthetic bacteria, and genes necessary for chloroplast function have moved from the chloroplast to the nuclear genome of the host eukaryotic cell. Proteins encoded by these genes are made in the cytosol and imported into the chloroplast using protein translocases. Chloroplasts contain the characterized SEC1 and the recently-discovered SEC2 translocase systems. The SEC2 translocase consists of the SECA2, SCY2, and SECE2 proteins. Since SEC2 was recently discovered, its protein substrates are unknown. Inducible RNAi targeting SCY2 revealed decreased levels of many candidate proteins upon silencing. To identify substrates for SEC2, Li, et al. developed an in vitro method to test protein integration into isolated chloroplast membranes. Using this assay, FTSH12 integration in the assay was inhibited by the SECA inhibitor sodium azide, which identified FTSH12 as a SEC2 substrate. TIC40 was identified as a second substrate because antibodies against epitope-tagged SCY2 and SECE2 inhibited TIC40 integration. (Review by
Chloroplasts have evolved from photosynthetic bacteria, and genes necessary for chloroplast function have moved from the chloroplast to the nuclear genome of the host eukaryotic cell. Proteins encoded by these genes are made in the cytosol and imported into the chloroplast using protein translocases. Chloroplasts contain the characterized SEC1 and the recently-discovered SEC2 translocase systems. The SEC2 translocase consists of the SECA2, SCY2, and SECE2 proteins. Since SEC2 was recently discovered, its protein substrates are unknown. Inducible RNAi targeting SCY2 revealed decreased levels of many candidate proteins upon silencing. To identify substrates for SEC2, Li, et al. developed an in vitro method to test protein integration into isolated chloroplast membranes. Using this assay, FTSH12 integration in the assay was inhibited by the SECA inhibitor sodium azide, which identified FTSH12 as a SEC2 substrate. TIC40 was identified as a second substrate because antibodies against epitope-tagged SCY2 and SECE2 inhibited TIC40 integration. (Review by