On the Inside: A Glucose-Dependent Feedback Loop Involved in Root Growth
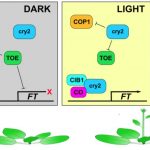
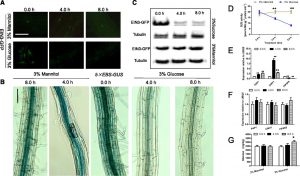 Sucrose is the major source of carbon for plant cells, and consequently sucrose catabolism in plants is one of the largest metabolic fluxes in primary carbon assimilation. However, sucrose cannot be directly utilized in plants. Therefore, after arriving at a sink, sucrose must be hydrolyzed. In the case of growing sinks as opposed to storage sinks, sucrose is hydrolyzed by invertase to form glucose and fructose. Loss-of-function mutations in cytosolic invertases (CINV) decrease root glucose levels, leading to reduced primary root growth, while exogenous glucose application promotes both primary and lateral root growth and root hair formation. In roots, glucose also controls the stability of the transcription factor Ethylene-Insensitive3 (EIN3) via the function of Hexokinase1 (HXK1), a glucose sensor. In Arabidopsis, Phosphatidylinositol monophosphate 5-kinase (PIP5K) isoforms have been identified and PIP5K9 has been shown to interact with and regulate CINV1 at a post-translational level by an unknown mechanism to regulate root growth and development. Anthocyanin Pigment 1 (PAP1), a transcription factor, is considered a key integration point for a variety of internal and external stimuli that influence the biosynthesis of anthocyanins. Significantly, sucrose, but not other sugars, can induce PAP1 expression. Meng et al. (10.1104/pp.20.00778) now demonstrate that in Arabidopsis EIN3 binds directly to both the promoters of PAP1 and PIP5K9, repressing and enhancing their expression, respectively. Subsequently, PAP1 binds directly to and promotes transcription from the CINV1 promoter, while PIP5K9 interacts with and negatively regulates CINV1. The accumulated CINV1 subsequently hydrolyzes sucrose, releasing the sequestered signaling cue, glucose, which has been shown to negatively regulate the stability of EIN3 via HXK1. The authors propose that a CINV1-glucose-HXK1-EIN3-PAP1/PIP5K9-CINV1 loop contributes to the modulation of CINV1 activity, thereby regulating root growth by glucose signaling.
Sucrose is the major source of carbon for plant cells, and consequently sucrose catabolism in plants is one of the largest metabolic fluxes in primary carbon assimilation. However, sucrose cannot be directly utilized in plants. Therefore, after arriving at a sink, sucrose must be hydrolyzed. In the case of growing sinks as opposed to storage sinks, sucrose is hydrolyzed by invertase to form glucose and fructose. Loss-of-function mutations in cytosolic invertases (CINV) decrease root glucose levels, leading to reduced primary root growth, while exogenous glucose application promotes both primary and lateral root growth and root hair formation. In roots, glucose also controls the stability of the transcription factor Ethylene-Insensitive3 (EIN3) via the function of Hexokinase1 (HXK1), a glucose sensor. In Arabidopsis, Phosphatidylinositol monophosphate 5-kinase (PIP5K) isoforms have been identified and PIP5K9 has been shown to interact with and regulate CINV1 at a post-translational level by an unknown mechanism to regulate root growth and development. Anthocyanin Pigment 1 (PAP1), a transcription factor, is considered a key integration point for a variety of internal and external stimuli that influence the biosynthesis of anthocyanins. Significantly, sucrose, but not other sugars, can induce PAP1 expression. Meng et al. (10.1104/pp.20.00778) now demonstrate that in Arabidopsis EIN3 binds directly to both the promoters of PAP1 and PIP5K9, repressing and enhancing their expression, respectively. Subsequently, PAP1 binds directly to and promotes transcription from the CINV1 promoter, while PIP5K9 interacts with and negatively regulates CINV1. The accumulated CINV1 subsequently hydrolyzes sucrose, releasing the sequestered signaling cue, glucose, which has been shown to negatively regulate the stability of EIN3 via HXK1. The authors propose that a CINV1-glucose-HXK1-EIN3-PAP1/PIP5K9-CINV1 loop contributes to the modulation of CINV1 activity, thereby regulating root growth by glucose signaling.