Novel Electrical Signaling: First Fast Voltage-Gated Sodium Channel Identified Outside of the Animal Kingdom
,
Electrical signals or action potentials are present in animals, plants, and other organisms such as single-celled diatoms (Moran et al., 2015; Hedrich et al., 2016; Helliwell et al., 2019). Electrical signals are short-lived, locally restricted changes in membrane potential (measured as voltage changes) that travel along cell membranes like waves. These signals are generated by the coordinated action of plasma membrane-localized ion channels, with specific characteristics crucial for generating and shaping electrical signals. The mechanisms used to generate electrical signals differ greatly between plants and animals. In animals, sodium (Na+) and potassium (K+) channels shape electrical signals, and the channel characteristics allow for extremely fast action potentials. These sodium channels, called NaVs, are voltage gated and able to activate and inactivate within milliseconds, and this gating speed is a key factor in electrical signaling in nerves and muscles (Moran et al., 2015). In land plants, electrical action potentials are slower (in the range of seconds) and are dependent on the activity of anion channels (for example, Cl− channels) and K+ channels. Na+ is not involved in electrical signaling in plants, and plants do not harbor voltage-dependent Na+ channels like those found in animal genomes; however, Ca2+ also plays a role in initiating electrical signals (Hedrich et al., 2016; Jezek and Blatt, 2017).
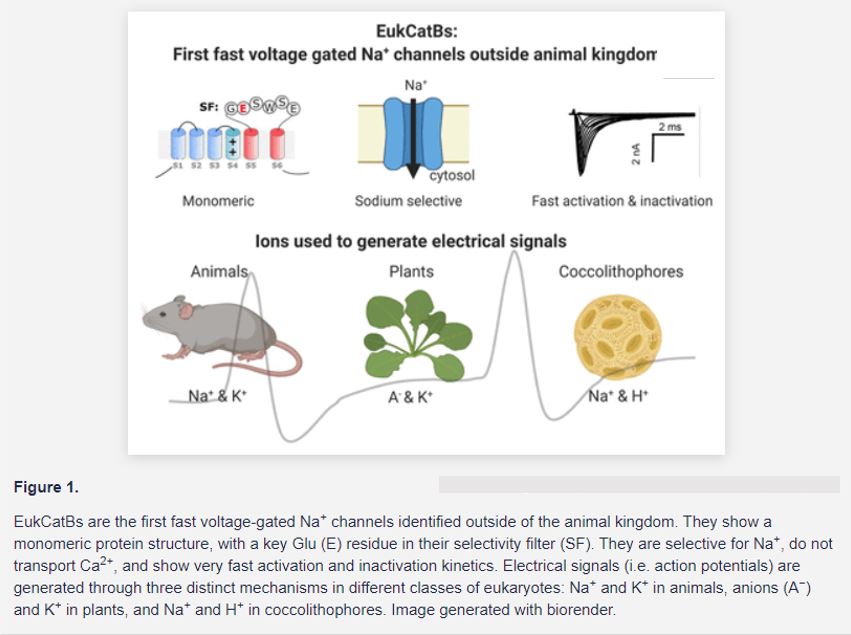 In this issue of Plant Physiology, Helliwell et al. (2020) identified the first Na+-selective channel outside of the animal kingdom. They characterized two novel members of a unique class of ion channels called EukCatBs, from Emiliania huxleyi and Scyphospaera apsteinii. These two unicellular eukaryotes are coccolithophores, marine plankton species that play an important ecological role in carbon fixation. Heterologous expression of the newly identified coccolithophore channels in human embryo kidney (HEK) cells demonstrated that they present the functional hallmarks required for generating a fast action potential. Like animal NaVs, EukCatBs show fast voltage-gating kinetics, with activation and inactivation occurring within the millisecond range (Fig. 1).
In this issue of Plant Physiology, Helliwell et al. (2020) identified the first Na+-selective channel outside of the animal kingdom. They characterized two novel members of a unique class of ion channels called EukCatBs, from Emiliania huxleyi and Scyphospaera apsteinii. These two unicellular eukaryotes are coccolithophores, marine plankton species that play an important ecological role in carbon fixation. Heterologous expression of the newly identified coccolithophore channels in human embryo kidney (HEK) cells demonstrated that they present the functional hallmarks required for generating a fast action potential. Like animal NaVs, EukCatBs show fast voltage-gating kinetics, with activation and inactivation occurring within the millisecond range (Fig. 1).
Helliwell et al. (2020) then further showed that the two coccolitophore EukCatBs are selective for Na+ and do not transport Ca2+. This separates them from the other EukCats previously characterized in diatoms, which are permeable to both Na+ and Ca2+ (Helliwell et al., 2019). Site-directed mutagenesis of a Glu (E) to an Asp (D) in the selectivity filter made EhEukCatB permeable to Na+ and Ca2+, suggesting that this specific amino acid in the selectivity filter motif is most likely responsible for Na+ selectivity (Fig. 1). This is particularly interesting, as one of the key features of animal NaVs, required for Na+ selectivity, is the arrangement of their protein domains (Moran et al., 2015). Animal NaVs consist of four pseudomonomers that are covalently linked. Importantly, the Na+ selectivity of animal NaVs depends on a specific combination of amino acids from each of the four subunit’s different selectivity filters. Differing from their animal counterparts, however, EukCatBs show a monomeric primary structure and might possibly form homotetramers. At the position of the Glu (E) in EukCatBs, animal NaVs harbor an Asp (D), Glu (E), lysin (K), and an Ala (A) residue, respectively, in their four subunits. This DEKA sequence is one of the key characteristics of animal NaVs and was thought to be crucial to achieve Na+ selectivity in similarly structured proteins (Moran et al., 2015). Its absence in coccolithophore EukCatBs demonstrates that other structural changes can also lead to Na+ selectivity and it will be interesting to further investigate this phenomenon.
After thorough characterization of the Na+-selective channels in HEK cells, Helliwell et al. (2020) then tested if the coccolithophores can generate fast Na+-dependent electrical signals at their plasma membrane. They performed patch-clamp experiments directly on S. apsteinii and showed that this eukaryote is capable of generating fast activating and inactivating Na+ currents when the membrane potential is modified. This strongly suggests that Na+ signaling occurs in coccolithophores and possibly in other haptophytes and that EukCatBs are major components of this signaling mechanism.
The function of these electrical signals in unicellular coccolithophores is currently elusive. Coccolithophores are characterized by a calcareous exoskeleton and possess two motile flagella and a third highly motile appendage, the haptonema. Electrical signaling might be involved in sensing environmental cues to guide movement toward a nutrient source or to coordinate the diurnal movements of the plankton species in the ocean. In addition to raising the question about the function of electrical signals in these eukaryotes, the findings by Helliwell et al. (2020) also offer a new perspective on electrical signaling. Previously, the authors had shown that coccolithophores use H+ instead of K+ to generate electrical signals (Taylor and Brownlee, 2003; Taylor et al., 2011). In combination with the new results, this demonstrates that the ions involved in shaping electrical signaling can be Na+ in combination with H+, rather than with K+. This sets coccolithophores apart from both animals and plants and reveals that a third distinct mechanism to generate action potentials evolved in eukaryotes (Fig. 1).
The reason why these different mechanisms for electrical signaling exist is an open question. These mechanisms might simply reflect different evolutionary outcomes to obtain the same beneficial cellular function of electrical signaling, which by chance took different pathways. It is also possible that the different ionic conditions faced by animal cells, plant cells, and marine coccolithophores might explain this different evolution. Understanding the processes utilizing electrical signaling in these organisms will provide important information regarding the biology of these marine organisms and the evolution of electrical signaling in the different kingdoms. This might reveal key features of ion channels and electrical signaling—knowledge that may spur medical, agricultural, and ecological advances.
REFERENCES
Hedrich R, Salvador-Recatalà V, Dreyer I. (2016) Electrical wiring and long-distance plant communcation Trends Plant Sci 21: 376–387
Helliwell KE, Chrachri A, Koester J, Wharam S, Taylor A, Wheeler G, Brownlee C (2020) A novel single-domain Na+-selective voltage-gated channel in photosynthetic eukaryotes. Plant Physiol 184: 1674–1683 https://doi.org/10.1104/pp.20.00889
Helliwell KE, Chrachri A, Koester JA, Wharam S. Verret F. Taylor AR, Wheeler GL, Brownlee C (2019) Alternative mechanisms for fast Na+/Ca2+ signaling in eukaryotes via a novel class of single-domain voltage-gated channels. Curr Biol 29: 1503–1511.e6
Jezek M, Blatt MR (2017) The membrane transport system of the guard cell and its integration for stomatal dynamics. Plant Physiol 174: 487–51
Moran Y, Barzilai MG, Liebeskind BJ, Zakon HH (2015) Evolution of voltage-gated ion channels at the emergence of Metazoa. J Exp Biol 218: 515–525
Taylor AR, Brownlee C (2003) A novel Cl− inward-rectifying current in the plasma membrane of the calcifying marine phytoplankton Coccolithus pelagicus. Plant Physiol 131: 1391–1400
Taylor AR, Chrachri A, Wheeler G, Goddard H, Brownlee C (2011) voltage-gated H+ channel underlying pH homeostasis in calcifying coccolithophores. PLoS Biol 9: e1001085