New Insights into Chloroplast Division
Chloroplasts divide by binary fission, a process that is driven by a ring-like multiprotein complex spanning the inner and outer envelope membranes (OEMs) at the site of division. DYNAMIN-RELATED PROTEIN 5B (DRP5B/ ARC5), 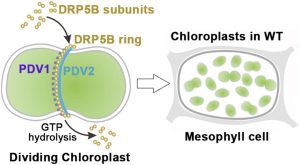 a cytosolic component of the chloroplast division machinery, is thought to function in the fission of chloroplast envelopes: this finding is consistent with the occurrence of enlarged, dumbbell-shaped chloroplasts in the mesophyll cells of arc5 mutants. Dynamin and dynamin-related proteins are mechanochemical GTPases that function via oligomerization and GTP-dependent conformational changes that exert forces on membranes, thereby promoting membrane fission. During chloroplast division, DRP5B subunits must be recruited from the cytosol to the chloroplast surface where they assemble into a ring-like structure (DRP5B ring) at the chloroplast division site, thereby facilitating fission of the chloroplast envelope. However, the molecular nature of DRP5B-membrane interactions and their regulation by guanine nucleotides and protein effectors remain poorly characterized. In Arabidopsis, PLASTID DIVISION1 (PDV1) and its paralog PDV2 are key components of the chloroplast division machinery in the OEM. Both proteins span the lipid bilayer via a single transmembrane domain and localize into ring-like structures at the chloroplast division site. Sun et al. (10.1104/pp.20.00199) provide evidence that DRP5B targeting to the chloroplast surface and assembly into a ring structure at the division site are specifically determined by the chloroplast outer OEM protein PLASTID DIVISION2 (PDV2), and that DRP5B-OEM dissociation, which is driven by GTP hydrolysis, is mediated mainly by PDV1, a paralog of PDV2. The authors conclude that the mechanochemical properties of DRP5B on the chloroplast surface are dynamically regulated by its GTPase activity and major binding partners.
a cytosolic component of the chloroplast division machinery, is thought to function in the fission of chloroplast envelopes: this finding is consistent with the occurrence of enlarged, dumbbell-shaped chloroplasts in the mesophyll cells of arc5 mutants. Dynamin and dynamin-related proteins are mechanochemical GTPases that function via oligomerization and GTP-dependent conformational changes that exert forces on membranes, thereby promoting membrane fission. During chloroplast division, DRP5B subunits must be recruited from the cytosol to the chloroplast surface where they assemble into a ring-like structure (DRP5B ring) at the chloroplast division site, thereby facilitating fission of the chloroplast envelope. However, the molecular nature of DRP5B-membrane interactions and their regulation by guanine nucleotides and protein effectors remain poorly characterized. In Arabidopsis, PLASTID DIVISION1 (PDV1) and its paralog PDV2 are key components of the chloroplast division machinery in the OEM. Both proteins span the lipid bilayer via a single transmembrane domain and localize into ring-like structures at the chloroplast division site. Sun et al. (10.1104/pp.20.00199) provide evidence that DRP5B targeting to the chloroplast surface and assembly into a ring structure at the division site are specifically determined by the chloroplast outer OEM protein PLASTID DIVISION2 (PDV2), and that DRP5B-OEM dissociation, which is driven by GTP hydrolysis, is mediated mainly by PDV1, a paralog of PDV2. The authors conclude that the mechanochemical properties of DRP5B on the chloroplast surface are dynamically regulated by its GTPase activity and major binding partners.



