MicroProteins as the First Step toward a Master Key for Posttranslational Regulation
Determining the function of a gene often relies on transgenic plants with altered transcriptional or translational levels. This is usually achieved through overexpression, knockout, or knockdown. However, each of these approaches has specific advantages and drawbacks. For instance, knockouts, which can be created through CRISPR-Cas9 technology, support complete loss-of-function approaches. These, therefore, are inadequate for essential genes. In such cases, knockdown methods, for example, by siRNA, retain a basal level of expression. However, both methods can have off-target effects. Currently, there are few methods for the regulation of proteins at the posttranslational level, and an extension of our biotechnology toolbox that facilitated control at this level would be a valuable alternative. One naturally occurring principle of posttranslational regulation is the recently discovered microProteins. These proteins consist of a single domain that facilitates protein-protein interactions. Through this domain, microProteins can disrupt the functions of larger protein complexes (Slavoff et al., 2013; Eguen et al., 2015).
In this issue of Plant Physiology, Dolde et al. (2018) report on microProteins in Arabidopsis (Arabidopsis thaliana) and discuss how to generate and use synthetic microProteins as a much-needed tool for posttranslational regulation in plants. Previously, this potential had been demonstrated only for transcription factors (Magnani et al., 2014). Now, Dolde et al. use three targets as a test set to show that microProteins can be used for the regulation of multiprotein complexes. The first example is DICER-LIKE1 (DCL1). Expression of the PIWI/ARGONAUTE/ZWILLE (PAZ) domain as a microProtein (miP-DCL1) led to abnormal growth phenotypes and, to the surprise of the authors, increased processing of miRNAs rather than decreased processing as predicted. The authors concluded that miP-DCL1 interacts with an unknown suppressor of DCL1 through the PAZ domain, thereby sequestering the suppressor and increasing the activity of DCL1. This observation demonstrates how results obtained with artificial microProteins could spark further research, for example, the identification of said suppressor. The second example represents down-regulation of an activity. Expression of the juxtamembrane domain of the BRASSINOSTEROID INSENSITIVE1 (BRI1; miP-BRI1) receptor generates mild dwarfing and brassinosteroid insensitivity. BRI1 and miP-BRI1 most likely form inactive dimers in the presence or absence of brassinosteroids (see Fig. 1). The third example again demonstrates loss of function, in this case through dimerization. The authors expressed the photolyase domain of CRYPTOCHROME1 (CRY1; miP-CRY1). Plants remained in an etiolated state after exposure to blue light, similar to a cry1cry2 double mutant. While the authors concluded that CRY1 is affected, off-target effects with CRY2 cannot be excluded.

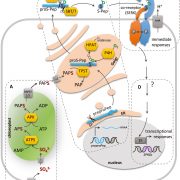
A model for microProtein function. The brassinosteroid receptor BRI1 is activated by binding to brassinolide (BL), causing dissociation of inhibitory BKI1 and binding of BRI1-ASSOCIATED RECEPTOR KINASE1 (BAK1). When present, the microProtein miP-BRI1 binds to BRI1 and prevents its activation. (From Dolde et al., 2018, figure 3A.)
Indeed, one current drawback of microProtein applications is the potential for off-target effects, which for now remain uncharted territory. Such off-target effects might prove to be less predictable than for siRNA or CRISPR-Cas9 approaches, as protein-protein interactions are more complex than interactions between nucleic acids.
Another characteristic of this microProtein technique is its reliance on highly specific protein-protein interactions. Therefore, this technology is currently limited to proteins with known protein-protein interaction domains. However, in vitro engineering efforts might allow us to design artificial and highly specific interactions, thereby adding a further design element for synthetic biology approaches. Excitingly, this method already allows us to manipulate protein complexes in selective ways, especially if tissue-specific or inducible promotors are used.
REFERENCES
Dolde U, Rodrigues V, Straub D, Bhati KK, Choi S, Yang SW, Wenkel S (2018) Synthetic microProteins: versatile tools for posttranslational regulation of target proteins. Plant Physiol 176: 3136–3145
Eguen T, Straub D, Graeff M, Wenkel S (2015) MicroProteins: small size-big impact. Trends Plant Sci 20: 477–482
Magnani E, de Klein N, Nam H-I, Kim J-G, Pham K, Fiume E, Mudgett MB, Rhee SY (2014) A comprehensive analysis of microProteins reveals their potentially widespread mechanism of transcriptional regulation. Plant Physiol 165: 149–159
Slavoff SA, Mitchell AJ, Schwaid AG, Cabili MN, Ma J, Levin JZ, Karger AD, Budnik BA, Rinn JL, Saghatelian A (2013) Peptidomic discovery of short open reading frame-encoded peptides in human cells. Nat Chem Biol 9: 59–64