MaXB3 Limits Ethylene Production and Ripening of Banana Fruits
Author: Sjon Hartman
ORCID: 0000-0002-6709-6436
Plant Ecophysiology, Institute of Environmental Biology, Utrecht University, Padualaan 8, 3584 CH, Utrecht, The Netherlands
j.g.w.hartman@uu.nl
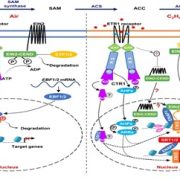
Ripening of climacteric fruits such as banana (Musa acuminata), tomato (Solanum lycopersicum), and mango (Mangifera indica) is typically triggered and regulated by the gaseous plant hormone ethylene (Seymour et al., 2013; Hu et al., 2019). As a gas, ethylene levels and subsequent signal transduction in plants can be regulated by changes in biosynthesis and gas diffusion rates (Abeles et al., 2012; Hartman et al., 2020). In climacteric fruits, ethylene signaling is activated through an initial burst in ethylene biosynthesis at the onset of the ripening process (Seymour et al., 2013). Ethylene biosynthesis can be summarized as a simple two-step reaction, where 1‐aminocyclopropane‐1‐carboxylic (ACC) synthase (ACS) controls the production of ACC, which in turn is converted to ethylene by ACC oxidase (ACO) (Pattyn et al., 2020) (Fig. 1A). While ACSs and ACOs are established regulators of ethylene biosynthesis and subsequent climacteric fruit ripening (Seymour et al., 2013), little is known about how these enzymes themselves are regulated to control ripening processes.
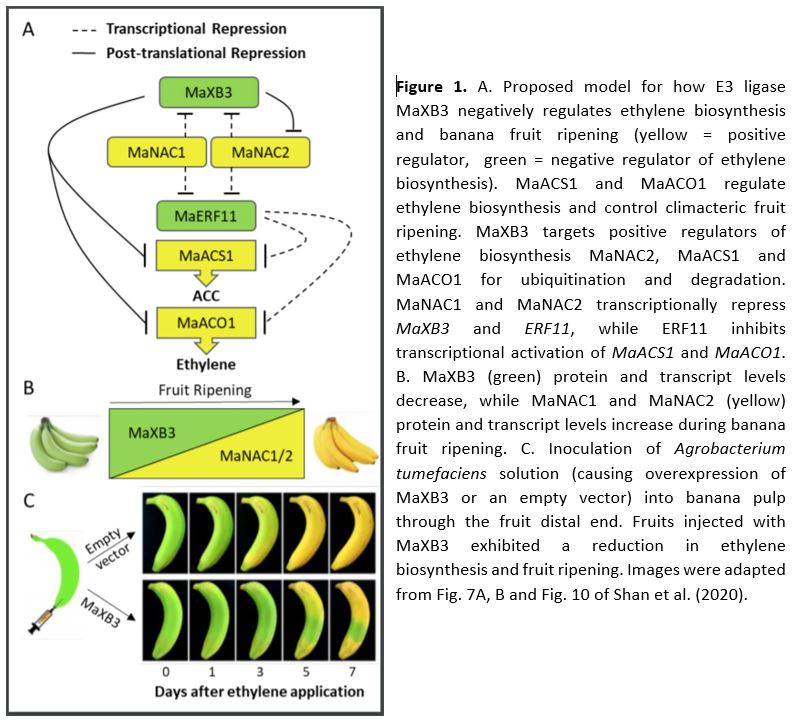 In this issue of Plant Physiology, Shan et al. (2020) report several genes involved in the transcriptional and post-translational control of ethylene biosynthesis and banana fruit ripening. The authors previously uncovered ETHYLENE RESPONSE FACTOR11 (MaERF11) as a transcriptional repressor of the ethylene biosynthesis genes MaACS1 and MaACO1 (Xiao et al., 2013). In the current study, they reveal that MaERF11 itself is also negatively regulated by the two NAC transcription factors, MaNAC1 and MaNAC2 (Shan et al., 2020). Using several techniques, they demonstrate that both MaNAC1 and MaNAC2 directly repress MaERF11 promoter activity. Together these findings reveal that MaNAC1/2 positively regulate ethylene biosynthesis and banana fruit ripening through inhibition of the transcriptional repressor ERF11 (Fig. 1A).
In this issue of Plant Physiology, Shan et al. (2020) report several genes involved in the transcriptional and post-translational control of ethylene biosynthesis and banana fruit ripening. The authors previously uncovered ETHYLENE RESPONSE FACTOR11 (MaERF11) as a transcriptional repressor of the ethylene biosynthesis genes MaACS1 and MaACO1 (Xiao et al., 2013). In the current study, they reveal that MaERF11 itself is also negatively regulated by the two NAC transcription factors, MaNAC1 and MaNAC2 (Shan et al., 2020). Using several techniques, they demonstrate that both MaNAC1 and MaNAC2 directly repress MaERF11 promoter activity. Together these findings reveal that MaNAC1/2 positively regulate ethylene biosynthesis and banana fruit ripening through inhibition of the transcriptional repressor ERF11 (Fig. 1A).
Next, the authors used yeast two-hybrid screens to search for putative MaNAC1/2 interacting proteins and uncovered the E3 ligase XA21 Binding Protein 3 (MaXB3) as a top candidate. Moreover, they found that MaXB3 transcript and protein levels decreased during fruit ripening, while MaNAC1/2 levels increased (Fig. 1B). This negative association suggested that MaXB3 and MaNAC1/2 could potentially balance each other’s levels to control fruit ripening. Previous research in rice and Arabidopsis classified MaXB3 orthologues as functional E3 ligases that flag their targets for ubiquitination, leading to proteasomal degradation (Wang et al., 2006; Prasad et al., 2010; Lyzenga et al., 2012). Therefore, the authors tested whether MaNAC1/2 levels are under MaXB3-mediated proteolytic control and found that MaXB3 indeed ubiquitinates and degrades MaNAC2, but not MaNAC1. Additionally, they showed that MaXB3 interacts with and ubiquitinates the essential ethylene biosynthesis enzymes MaACS1 and MaACO1, and causes their subsequent proteasomal degradation. Collectively, the results of Shan et al. (2020) show that MaXB3 promotes MaACS1, MaACO1 and MaNAC2 degradation and potentially limits ethylene biosynthesis and fruit ripening in banana (Fig. 1A).
Since MaXB3 levels typically decline as the fruits ripen (Fig. 1B), the authors wondered if ectopic overexpression of MaXB3 would lead to a delay in ethylene production and subsequent ripening. To test this, MaXB3 was transiently overexpressed in unripe bananas. MaXB3 overexpression indeed led to a local reduction in MaNAC2, MaACS1, MaACO1, ethylene biosynthesis, and ethylene-responsive gene expression and a delay in ripening (Fig. 1C). Finally, the authors heterologously expressed MaXB3 in tomato plants and again found that elevated MaXB3 levels led to a reduction of ethylene biosynthesis, signaling and ripening in tomato fruits. These experiments suggest that MaXB3 function is also conserved in other climacteric fruit species. Taken together, the work of Shan et al. (2020) identifies MaXB3 as an essential negative regulator of ethylene biosynthesis and climacteric fruit ripening.
Several questions remain about how MaXB3 is normally regulated during fruit development and subsequent ripening. Shan et al. (2020) show that MaXB3 is transcriptionally repressed by increasing levels of MaNAC1/2 and ethylene (Fig. 1A), which could function as a feedback loop to stimulate the ripening process once MaNAC1/2 and ethylene levels increase (Fig. 1A, B). Future research could aim to make the MaXB3 promoter less sensitive to this MaNAC1/2 repression through binding site deletion, with the potential to slow down fruit ripening. Conversely, what if MaXB3 could already be experimentally repressed during fruit development, would this lead to premature and/or accelerated fruit ripening? Finally, how are high levels of MaXB3 initially maintained during fruit development (Fig. 1B), and can they be altered by developmental and environmental signals? Currently, inadequate control over fruit ripening processes contributes to large amounts of food waste globally (Hu et al., 2019). One promising approach may therefore be to identify regulators of MaXB3 in the future, as increased control over MaXB3 levels could ultimately allow breeders, retailers and consumers to delay ripening and increase the shelf life of our beloved fruits.
Literature Cited
Abeles FB, Morgan PW, Saltveit ME (2012) Ethylene in Plant Biology: Second Edition. Ethyl Plant Biol Second Ed. doi: 10.1016/C2009-0-03226-7
Hartman S, Sasidharan R, Voesenek LACJ (2020) The role of ethylene in metabolic acclimations to low oxygen. New Phytol nph.16378
Hu B, Sun DW, Pu H, Wei Q (2019) Recent advances in detecting and regulating ethylene concentrations for shelf-life extension and maturity control of fruit: A review. Trends Food Sci Technol 91: 66–82
Lyzenga WJ, Booth JK, Stone SL (2012) The Arabidopsis RING-type E3 ligase XBAT32 mediates the proteasomal degradation of the ethylene biosynthetic enzyme, 1-aminocyclopropane-1- carboxylate synthase 7. Plant J 71: 23–34
Pattyn J, Vaughan‐Hirsch J, Van de Poel B (2020) The regulation of ethylene biosynthesis: a complex multilevel control circuitry. New Phytol nph.16873
Prasad ME, Schofield A, Lyzenga W, Liu H, Stone SL (2010) Arabidopsis RING E3 ligase XBAT32 regulates lateral root production through its role in ethylene biosynthesis. Plant Physiol 153: 1587–1596
Seymour GB, Østergaard L, Chapman NH, Knapp S, Martin C (2013) Fruit Development and Ripening. Annu Rev Plant Biol 64: 219–241
Wang YS, Pi LY, Chen X, Chakrabarty PK, Jiang J, De Leon AL, Liu GZ, Li A, Benny U, Oard J, et al (2006) Rice XA21 binding protein 3 is a ubiquitin ligase required for full Xa21-mediated disease resistance. Plant Cell 18: 3635–3646
Xiao YY, Chen JY, Kuang JF, Shan W, Xie H, Jiang YM, Lu WJ (2013) Banana ethylene response factors are involved in fruit ripening through their interactions with ethylene biosynthesis genes. J Exp Bot 64: 2499–2510