LACCASE8: An Important Component of a Gene Toolkit for Engineering C-lignin in Crops
Wang et al. uncover the role of Laccase8 from the model system Cleome hassleriana in initiating C-lignin polymerization. The Plant Cell (2020) https://doi.org/10.1105/tpc.20.00598
By Xin Wanga,b,, Chunliu Zhuoa,c, and Richard A. Dixona
aUniversity of North Texas, 1155 Union Circle #311428, Denton, TX 76203, USA
b Oil Crops Research Institute of the Chinese Academy of Agricultural Sciences, Wuhan, China
cCenter for Bioenergy Innovation (CBI), Oak Ridge National Laboratory, Oak Ridge, TN 37831, USA
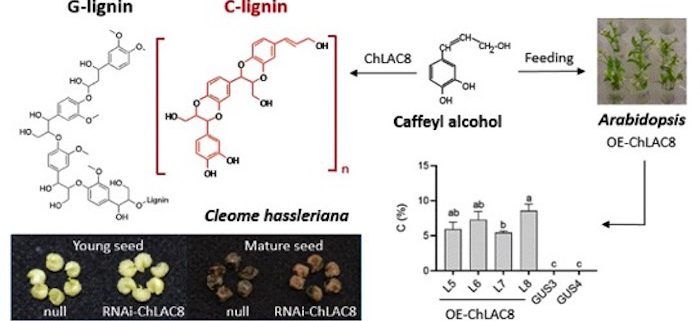
Background: Lignin, the most abundant aromatic polymer on Earth, strengthens and waterproofs plant secondary cell walls. For a long time, lignin was believed to be built from only three different units known as monolignols. The polymerization of monolignols to lignin is catalyzed by two groups of enzyme systems: laccases and peroxidases. In 2012, a new type of lignin (named C-lignin) was discovered by our group in the seed coats of diverse plant species, such as the vanilla orchid Vanilla planifolia and many members of the Cactaceae. C-lignin is made from a new monolignol, caffeyl alcohol, with a single type of linkage, and is also a linear molecule, making it an ideal bio-based material for carbon fibers and high-value chemicals. How caffeyl alcohol is polymerized to C-lignin was unknown.
Question: Does an enzyme exists that is specifically responsible for the polymerization of caffeyl alcohol into C-lignin? If so, what is its exact function? We tested this in an ornamental plant, Cleome hassleriana, with a unique lignin accumulation pattern in the seed coat, where biosynthesis of “classical” G-lignin (made from the monolignol coniferyl alcohol) switches to C-lignin biosynthesis at around 12 days after pollination.
Findings: We identified a seed coat-specific laccase enzyme from Cleome (ChLAC8). The expression profile of ChLAC8 parallels the accumulation pattern of C-lignin during seed maturation. ChLAC8 protein could specifically oxidize caffeyl alcohol rather than coniferyl alcohol. Suppression of ChLAC8 expression resulted in significantly reduced C-lignin content but no change in G-lignin content in the seed coats of transgenic Cleome plants. Expression of ChLAC8 in the model plant Arabidopsis thaliana allowed it to accumulate C-lignin if caffeyl alcohol was supplied.
Next steps: Scientists aim to produce high amounts of C-lignin in industrial plants as a high-value bioproduct. If we can genetically engineer sufficient levels of caffeyl alcohol in vegetative tissues, such as vessels and fibers, ChLAC8 will be an important component of a gene toolkit for introducing C-lignin in commercial biomass crops such as switchgrass and poplar.