Loose-Knit Family: Tracing the Evolution of Actin Depolymerizing Factors that Sever or Join the Actin Cytoskeleton
IN BRIEF by Jennifer Lockhart [email protected]
 The interior of a plant cell is supported by the actin cytoskeleton, a complex network of yarn-like fibers whose form changes as the cell develops, grows, and divides. Actin fibers readily come apart (sever) and join back together, depending on the needs of the cell. This process is carefully orchestrated by a myriad of proteins, such as actin depolymerizing factors (ADFs). ADFs can bind to both monomeric and filamentous actin (F-actin). Across the plant kingdom, ADFs play diverse, often unexplored roles in shaping the vegetative and reproductive actin cytoskeleton systems. The 11 ADFs of Arabidopsis thaliana have various tissue-specific gene expression patterns and activities. For example, ADF1, ADF2, ADF4, and ADF7 can sever actin filaments in vitro, whereas ADF9 shows actin-bundling and -stabilizing activity, especially at low pH (Tholl et al., 2011). Such findings prompted the intriguing hypothesis that ADF genes arose from a common ancestor through duplication and acquired diverse functions as actin fibers evolved (Kandasamy et al., 2007). The functional differences among ADFs might be due to changes in key amino acids or functional motifs, a challenging topic ripe for exploration.
The interior of a plant cell is supported by the actin cytoskeleton, a complex network of yarn-like fibers whose form changes as the cell develops, grows, and divides. Actin fibers readily come apart (sever) and join back together, depending on the needs of the cell. This process is carefully orchestrated by a myriad of proteins, such as actin depolymerizing factors (ADFs). ADFs can bind to both monomeric and filamentous actin (F-actin). Across the plant kingdom, ADFs play diverse, often unexplored roles in shaping the vegetative and reproductive actin cytoskeleton systems. The 11 ADFs of Arabidopsis thaliana have various tissue-specific gene expression patterns and activities. For example, ADF1, ADF2, ADF4, and ADF7 can sever actin filaments in vitro, whereas ADF9 shows actin-bundling and -stabilizing activity, especially at low pH (Tholl et al., 2011). Such findings prompted the intriguing hypothesis that ADF genes arose from a common ancestor through duplication and acquired diverse functions as actin fibers evolved (Kandasamy et al., 2007). The functional differences among ADFs might be due to changes in key amino acids or functional motifs, a challenging topic ripe for exploration.
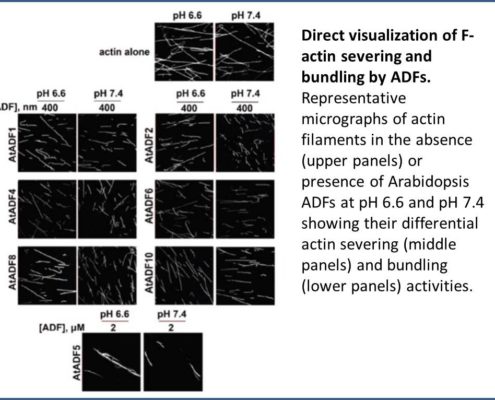
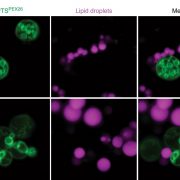
Nan et al. (2017) rose to the challenge by exploring the evolution of ADF family members across various plant lineages and the amino acid changes that led to the diversification of their functions. The study began with an analysis of the effects of all 11 Arabidopsis ADFs on actin filaments in vitro based on a simple principle: Actin filaments are generally sedimented by high-speed centrifugation, whereas filaments severed by ADFs remain in the supernatant, especially at high pH. Most ADFs exhibited F-actin-severing activity. By contrast, low-speed co-sedimentation assays, which separated single actin filaments from actin filament bundles, revealed the F-actin bundling activity of ADF5 and ADF9. Fluorescence microscopy confirmed the diverse roles of the ADFs (see figure). The ADFs were divided into two categories based on activity: 1) D-type (depolymerizing F-actin) ADFs, which sever or depolymerize F-actin and 2) B-type (bundling F-actin) ADFs, which bind to and bundle F-actin. Phylogenetic analysis of ADFs across the plant kingdom suggested that an intron-sliding event (movement of an intron from one position in a gene to another) occurred during evolution. It appears that the ADF genes descended from a D-type common ancestor and expanded through various types of gene duplication events, which increased the chances of retaining these vital genes. The ADF genes were grouped into four ancient subclasses that have been conserved in angiosperms for approximately 250 million years. While most ADFs have completely retained the D-type function of their ancestors, a few B-type ADFs have completely lost this function and have undergone neo-functionalization.
The authors zeroed in on amino acid changes that led to the conversion of the original D-type function to B-type function by analyzing the F-actin severing and bundling activities of mutant, ancestral-like proteins. They identified several pivotal amino acid residues affecting the biochemical functions of these proteins; these residues are conserved in Arabidopsis and several other angiosperms. N-terminal extensions that arose from intron sliding events were found to be crucial for the change from D-type to B-type function. This mechanism appears to have remained fixed since the diversification of the angiosperm lineage, shedding light on this not so tight-knit family.
REFERENCES
Kandasamy, M.K., Burgos-Rivera, B., McKinney, E.C., Ruzicka, D.R., and Meagher, R.B. (2007). Class-specific interaction of profilin and ADF isovariants with actin in the regulation of plant development. Plant Cell 19: 3111-3126.
Nan, Q., Qian, D., Niu, Y., He, Y., Tong, S., Niu, Z., Ma, J., Yang, Y., An, L., Wan, D., and Xiang, Y. (2017). Plant actin-depolymerizing factors possess opposing biochemical properties arising from key amino acid changes throughout evolution. Plant Cell 29: doi:10.1015/tpc16.00690.
Tholl, S., Moreau, F., Hoffmann, C., Arumugam, K., Dieterle, M., Moes, D., Neumann, K., Steinmetz, A., and Thomas, C. (2011). Arabidopsis actin-depolymerizing factors (ADFs) 1 and 9 display antagonist activities. FEBS Lett. 585: 1821-1827.
FIGURE LEGEND
Direct visualization of F-actin severing and bundling by ADFs. Representative micrographs of actin filaments in the absence (upper panels) or presence of Arabidopsis ADFs at pH 6.6 and pH 7.4 showing their differential actin severing (middle panels) and bundling (lower panels) activities. (Adapted from Nan et al. [2017], Supplemental Figure 5A.)



Leave a Reply
Want to join the discussion?Feel free to contribute!