Leaf position makes a difference: the ABCB19 auxin transporter affects light perception
Author: David S. Favero1 (ORCID: 0000-0002-6879-0323)
Affiliation:
1RIKEN Center for Sustainable Resource Science, Yokohama, Kanagawa, 230-0045 Japan
Main text:
As autotrophs, plants must maintain photosynthesis to thrive. In order for this to happen, a plant’s chlorophyll must absorb sufficient sunlight to efficiently drive the light-dependent photosynthetic reactions. Most photosynthesis occurs in leaves, which possess a high surface area that facilitates light capture. The quantity of light absorbed by a leaf is affected by its morphology and position, and accordingly, these leaf properties are both precisely controlled by the plant. For instance, it has been demonstrated that blue light, acting via the phototropin1 (phot1) and phot2 photoreceptors, promotes light perception in Arabidopsis thaliana (Arabidopsis) by inducing leaf flattening and mild hyponasty, or the growth of petioles at an upright angle (Sakai et al., 2001; Takemiya et al., 2005; Inoue et al., 2008). Accordingly, plants grown in dim monochromatic red light accumulate much less biomass than those also supplemented with a low level of blue light, while this response is greatly compromised in mutants that are unable to properly manipulate leaf morphology and position in response to phototropin-derived signals (Takemiya et al., 2005; Inoue et al., 2008). Despite the importance of these adaptations for robust plant growth, the signaling mechanisms regulating such leaf responses are not clearly understood.
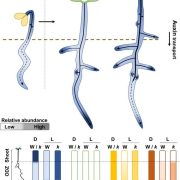
 In this issue of Plant Physiology, Jenness et al. (2020) identify the auxin transporter ABCB19 as a key regulator of leaf position downstream of the phototropins. While previous work had demonstrated that phot1-mediated inactivation of ABCB19 is important for phototropic responses in seedlings (Christie et al., 2011), it remained unknown if this auxin transporter regulates leaf position. Therefore, the authors of the current study first examined petiole positions in an abcb19 loss-of-function mutant (Jenness et al., 2020). They observed that abcb19 petioles are constitutively hyponastic and exhibit little response to different light treatments. This contrasts with the phot1 phot2 double mutant, in which petioles are positioned at a near-horizontal angle even when exposed to blue light (Inoue et al., 2008; Jenness et al., 2020).
In this issue of Plant Physiology, Jenness et al. (2020) identify the auxin transporter ABCB19 as a key regulator of leaf position downstream of the phototropins. While previous work had demonstrated that phot1-mediated inactivation of ABCB19 is important for phototropic responses in seedlings (Christie et al., 2011), it remained unknown if this auxin transporter regulates leaf position. Therefore, the authors of the current study first examined petiole positions in an abcb19 loss-of-function mutant (Jenness et al., 2020). They observed that abcb19 petioles are constitutively hyponastic and exhibit little response to different light treatments. This contrasts with the phot1 phot2 double mutant, in which petioles are positioned at a near-horizontal angle even when exposed to blue light (Inoue et al., 2008; Jenness et al., 2020).
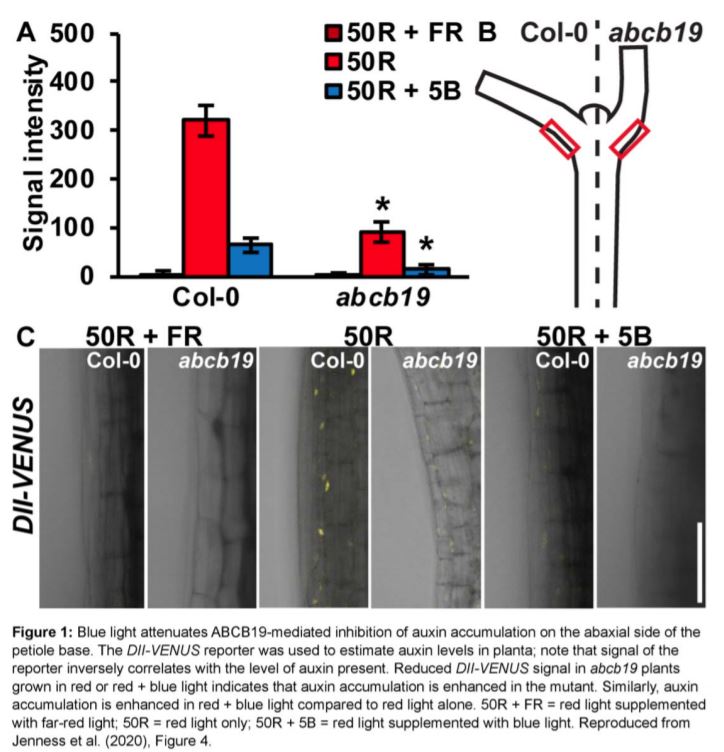
The authors next investigated if ABCB19 influences auxin accumulation at the base of the petiole, as this could explain its effect on leaf position. Hyponastic responses induced by a low ratio of red:far-red light, as experienced by plants growing in close proximity to each other, are caused by accumulation of auxin on the abaxial side of the petiole base, where it presumably induces cell division (Figure 1; Pantazopoulou et al., 2017; Jenness et al., 2020). Using the DII-VENUS reporter system to estimate auxin levels in the plant, Jenness et al. (2020) observed significantly greater auxin accumulation on the abaxial side petiole base of abcb19 compared to the wildtype for plants exposed to either red or red plus blue light (Figure 1). This indicates that increased auxin accumulation at the base of the petiole in abcb19 likely causes the constitutive hyponasty phenotype observed in this mutant. Additionally, the authors also found that blue light exposure increases auxin accumulation at the petiole base (Figure 1), suggesting that phototropin-mediated deactivation of abcb19 leads to auxin accumulation in this region, which consequently promotes hyponastic leaf movement.
One interesting question arising from this study is if phot1 and phot2 promote leaf hyponasty via similar mechanisms. Although phot1 predominantly regulates blue light-induced hyponasty, it is clear that phot2 plays a minor role in this response (Inoue et al., 2008; Jenness et al., 2020). Specifically, Jenness et al. (2020) found that phot2 exhibits decreased hyponasty only at the higher of two different blue fluence rates tested in this study. Contrastingly, phot1 alone promotes hyponasty in response to low blue light, while it acts semi-redundantly with phot2 when the blue fluence rate is higher (Inoue et al., 2008; Jenness et al., 2020). These genetic data suggest that the two phototropins might regulate leaf movement via similar mechanisms. However, while it is known that blue light-activated phot1 phosphorylates ABCB19, thus inhibiting the latter’s auxin transport function (Christie et al., 2011), it is unknown if phot2 regulates ABCB19 activity. Therefore, additional work is needed to understand if phot2 promotes blue light-induced hyponasty by inhibiting ABCB19 or via a different pathway.
Collectively, the data from Jenness et al. (2020) indicate that ABCB19 inhibits leaf hyponasty by suppressing auxin accumulation at the base of the petiole, while phot1, potentially acting together with phot2, partially relieves this inhibition. However, it remains unknown how phototropin-mediated inhibition of ABCB19 promotes auxin accumulation at the base of the petiole. A simple hypothesis is that ABCB19 located in this region is deactivated by phototropins, consequently reducing transport of auxin away from this location. ABCB19 might reduce auxin accumulation at the base of the petiole by facilitating its transport to a more basal location in the plant, as polar auxin transport from the shoot apex to the middle of the hypocotyl is reduced in abcb19 seedlings (Christie et al., 2011). An alternative hypothesis is that phototropin-mediated deactivation of ABCB19, rather than directly affecting auxin transport away from the base of the petiole, instead promotes
accumulation of auxin in this region indirectly by increasing total quantity of the hormone in the plant. Consistent with this hypothesis, Jenness et al. (2020) observed that free indole-3-acetic acid (IAA) levels are increased by approximately 15-20% in aerial portions of plants exposed to blue light, in a manner dependent on phototropins. While additional work is needed to establish the precise mechanism by which deactivation of ABCB19 leads to auxin accumulation at the base of petiole, it is now clear that this auxin transporter plays a key role regulating leaf position, and consequently, a plant’s ability to maximize light perception.
LITERATURE CITED
Christie JM, Yang H, Richter GL, Sullivan S, Thomson CE, Lin J, Titapiwatanakun B, Ennis M, Kaiserli E, Lee OR, Adamec J, Peer WA, Murphy AS (2011) phot1 inhibition of ABCB19 primes lateral auxin fluxes in the shoot apex required for phototropism. PLoS Biol 9: e1001076
Inoue S, Kinoshita T, Takemiya A, Doi M, Shimazaki K (2008) Leaf positioning of Arabidopsis in response to blue light. Molecular Plant 1: 15-26
Jenness MK, Tayengwa R, Murphy AS (2020) An ATP-binding cassette transporter, ABCB19, regulates leaf position and morphology during phototropin1-mediated blue light responses Plant Physiol https://doi.org/10.1104/pp.20.00223
Pantazopoulou CK, Bongers FJ, Kupers JJ, Reinen E, Das D, Evers JB, Anten NPR, Pierik R (2017) Neighbor detection at the leaf tip adaptively regulates upward leaf movement through spatial auxin dynamics. Proc Natl Acad Sci U S A 114: 7450-7455
Sakai T, Kagawa T, Kasahara M, Swartz TE, Christie JM, Briggs WR, Wada M, Okada K (2001) Arabidopsis nph1 and npl1: blue light receptors that mediate both phototropism and chloroplast relocation. Proc Natl Acad Sci U S A 98: 6969-6974
Takemiya A, Inoue S, Doi M, Kinoshita T, Shimazaki K (2005) Phototropins promote plant growth in response to blue light in low light environments. Plant Cell 17: 1120-1127